INTRODUCTION
Non-bacterial gastroenteritis is a major health problem worldwide. With the application of new and sensitive diagnostic techniques, norovirus (NoV) is now recognized as the leading pathogen causing acute non-bacterial gastroenteritis in humans [Reference Fang1]. NoV belongs to the Caliciviridae family. Of the five genotype groups (GG) of NoV, three (GI, GII, GIV) cause human illness. Based on the nucleic acid sequences of genes encoding the major capsid protein, there are eight genotypes in GI, 17 in GII, and one in GIV [Reference Zheng2]. Many studies have shown that the GII.4 genotype is the main cause of NoV gastroenteritis in humans [Reference Koek3–Reference Reuter, Pankovics and Szücs7].
Contaminated water is one of the most important vehicles for NoV gastroenteritis outbreaks globally [Reference Maunula, Miettinen and von Bonsdorff8, Reference Hewitt9]. Detecting NoV RNA in water suspected of causing illness helps confirm that a gastroenteritis outbreak is due to NoV. Of the 41 waterborne gastrointestinal disease outbreaks reported during 1998–2003 in Finland, water specimens from 28 outbreaks were available for analysis; 10 of these were attributed to NoV [Reference Maunula, Miettinen and von Bonsdorff8]. Several other countries have also reported waterborne NoV outbreaks [Reference Koek3, Reference Lysén10].
Most waterborne NoV gastroenteritis outbreaks have been attributed to genotype GI [Reference Hewitt9–Reference Kim11], although genotypes GII.3, GII.6 [Reference Parshionikar12], and GII.4 have been implicated. In September 2009, a gastroenteritis outbreak occurred in a small town school in Guangdong Province. Guangdong is a semi-tropical province located in southern China, with an estimated 110 million permanent and migrant residents. We report here the epidemiological, environmental, and laboratory investigation that identified NoV GII.4 contaminated well water as the source of this outbreak.
MATERIALS AND METHODS
Outbreak description
On 4 September 2009, an outbreak of gastroenteritis occurred in a school located in a small town in Guangdong Province. The school had 285 teachers, of whom 55% resided at the school; 50% of these dined in the school's restaurant; 5484 students (99% residing at the school, 99% dining in the school's restaurant), and 85 staff members for cleaning and security. There had been no rain or large-scale gatherings at the school in the 2 weeks before the outbreak. About 80% of the school's daily water supply – water that was used for all purposes except drinking – was from a single well. Water for drinking was supplied through a municipal water supply and was boiled first.
Epidemiological investigation
Cases were defined as patients with ⩾3 loose stools and/or vomiting in a 24-h period beginning from 31 August to 10 September, 2009. A retrospective cohort investigation using a standardized questionnaire was conducted on 10 September 2009, with 5795 people interviewed in-person and 59 by phone, with 100% enrolment. The exposed cohort was defined as those only served by water from the school well and the unexposed cohort was defined as all persons served only by the municipal water supply. Attack rates, relative risks (RR), and 95% confidence intervals (CI) were calculated using SPSS v. 13.0 (SPSS Inc., USA). A P value of <0·05 was considered statistically significant. Because NoV is also able to be transmitted through contaminated food, we also investigated common food exposures in students and teachers.
Environmental investigation
We examined the well construction log, current well and chlorination conditions, and potential sources of contamination. The township Centre for Disease Control (CDC) tested the well water for total aerobic bacteria and faecal coliforms using the most probable number (MPN) technique. Standard laboratory methods were used for bacteriological investigations.
Diarrhoeal specimen collection and processing
Diarrhoeal specimens were collected on 8 and 10 September 2009, from patients meeting the case definition; faecal and rectal swabs were collected from 19 students and nine teachers. Faecal specimens were diluted with Hank's solution to make a 10–20% w/v suspension. RNA extraction was performed after centrifugation (926 g for 2 min) to clarify supernatants. Rectal swabs were placed in Hank's solution vortexed for 2 min, subseqently discarding the swabs. Rectal swab suspensions were centrifuged (926 g for 3 min) to clarify the supernatant before RNA extraction.
Water specimen collection and processing
Water samples ranging in volume from 0·8 to 2·5 l were collected directly from the well (n = 1) and from tap water pumped from the well (n = 2) on 10 September 2009, prior to chlorination. On 16 September, after chlorination of the well by the township CDC, additional water samples were collected directly from the well (n = 1), from tap water pumped from the well (n = 2), from a storage tank holding well water (n = 1), from municipal tap water (n = 1) and from river water (n = 1). MgCl2 was added to the water specimens to make a final concentration of 0·05 m, and the pH was adjusted to 3·0. Water specimens were filtered through a mixed cellulose ester membrane [Reference Zhang13] (Advantec, USA) and eluted with buffer containing 0·05 m glycine and 3% beef extract (pH 9·5). Addition of 40% PEG6000 and 5 m NaCl (final concentration 10% and 0·3 m, respectively) was followed by incubation (12 h at 4 °C) and centrifugation (10 286 g for 30 min). Pellets were re-suspended in 1 ml of RNase-free water (Takara, Japan).
RNA extraction
Viral RNA was extracted from faecal and water samples using a QIAamp Viral RNA Mini kit (Qiagen, Germany) according to the manufacturer's protocol.
Absolute and quantitative real-time RT–PCR detection
For detection of NoV, we used primers COG2F/COG2R as described previously [Reference Kageyama14]. Real-time RT–PCR was conducted in an ABI.7500 Fast Real-Time PCR System (Applied Biosystems Inc., USA) using Perfect Real Time reagent (Takara). Real-time RT–PCR was performed in a 20 μl volume as described previously [Reference Kageyama14]. Fluorescent signals were recorded at 72 °C. Standard curves for determining the amount of virus in water specimens were generated by serially diluting plasmids containing NoV GI and GII PCR products. The formula Ct = 40·991 − 3·494 × log x (where Ct is the cycle threshold and x represents the viral quantity, copies/μl) was used for quantitative analysis of NoV. The correlation coefficient of the curve was 0·998. The percentage recovery, which is equal to the quantity of virus after concentration divided by the quantity of virus before concentration, could then be calculated.
NoV genotyping by nucleotide sequence analysis
Amplified products from two students, two teachers and the well-water specimens were selected for nucleotide sequence analysis. The primers JV13I/JV12Y and SKF/SKR designed by Vennema et al. [Reference Vennema, de Bruin and Koopmans15] and Kojima et al. [Reference Kojima16] were used to amplify portions of the genes encoding the RNA-dependent RNA polymerase (RdRp, nt 4279–4605) and capsid protein (nt 5046–5398), respectively. Bidirectional sequencing of the amplified PCR products was achieved using BigDye 3·1 chemistry (Applied Biosystems Inc.) using an ABI PRISM 3130 DNA analyser. DNA sequences were aligned using the Bioedit program and construction of phylogenetic trees was performed using MEGA 4.0 software. Reference viral gene sequences were provided by National Institute for Public Health and the Environment, The Netherlands. Nucleotide sequences were assigned Genbank accession numbers HM627865–HM627869 for RdRp, and HM627870–HM627874 for capsid sequences.
RESULTS
Epidemiological investigation
The first case of gastroenteritis occurred on 4 September 2009, with the peak incidence noted on 6 September (Fig. 1). The overall attack rate during this outbreak was 1·8% with 1·7% (92/5484) of students and 5·6% (16/285) of teachers affected. In total, 2·0% (62/3153) of the entire male, and 1·8% (46/2616) of the female school population were affected. The outbreak did not extend to the adjacent residential area, where the incidence of diarrhoea remained stable. Symptoms included diarrhoea (100%), abdominal pain (82%), vomiting (72%), nausea (61%), flatulence (51%), and fever (38%). In most cases, symptoms were mild, self-limiting, and lasted a median of 2 days. Some of the rooms (serving 2320 people) in the school complex received only untreated well water, other rooms (serving 1373 people) received only municipal tap water; the remaining rooms received water from both sources. An investigation revealed that all foods were boiled with municipal tap water but cleaned with untreated well water before cooking. Students, teachers and staff consumed the same foods. No risk factor related to food consumption was identified. Students and teachers reported drinking boiled tap water. The epidemiological investigation showed that the attack rates in the dormitory building supplied with untreated well water and the dormitory building supplied with municipal tap water were 2·0% and 1·1%, respectively. Exposure to well water had a RR of 1·9 (95% CI 1·1–3·4), indicating that well water was a risk factor for the outbreak. Well water was disinfected with chlorine on 10 September; no new cases were reported after 12 September (Fig. 1).

Fig. 1 [colour online]. Date and times of illness onset for cases associated with a norovirus outbreak in Guangdong, China, 2009.
Environmental investigation
Several sources of water to the school were investigated for NoV contamination. Routinely, untreated well water was first pumped into a storage tank and, from there, pumped to rooms through pipes as required. The well supplying this water was approximately 30 m deep; a drainage line at the bottom of the well leads to a place 20 m lower than the bottom of a nearby river. The well surface was completely covered, and the environment in the immediate vicinity was cluttered with debris. Microbiological investigation of the well water indicated faecal contamination: there were 2·2 × 103 colony-forming units (c.f.u.)/ml of aerobic bacteria and a large number of faecal coliforms, with an MPN count of 72/100 ml. Further environmental investigation was unable to determine the source of contamination.
Real-time RT–PCR results of the patient and water specimens
RNA samples from the students (8/19, 42%) and teachers (5/9, 56%) tested positive for NoV. Samples from the well (well water and pumped well water) were also positive for NoV by real-time RT–PCR before disinfection with chlorine (Table 1). After disinfection, well water remained positive although much weaker than before (Fig. 2); however, the storage tank and pumped water from the well tested negative. Municipal tap water and river water were negative for NoV (Table 1).

Fig. 2 [colour online]. Real-time RT–PCR result of water samples after disinfection.
Table 1. Real-time RT–PCR detection of norovirus RNA in well-water samples

* The source of the water is from the well, tank water is well water that was pumped to a holding tank; tap water indicates water pumped from the tank into the school water supply on demand.
† Well-water specimens were much weaker than before.
Quantification of NoV in water specimens
We detected 215 copies/μl of NoV in well water and water pumped from the well (tap water samples 1 and 2) before sample concentration. After 2500× concentration through a mixed cellulose ester membrane, up to 105 copies/μl NoV could be detected. Two pumped well-water samples (tap water samples 1 and 2) that were concentrated 800 times contained between 2·5 × 103 and 5·0 × 103 copies/μl, respectively (Table 2). Concentrating municipal or river water did not show a positive result in detection of NoV.
Table 2. Quantity and yields of norovirus recovered from water samples
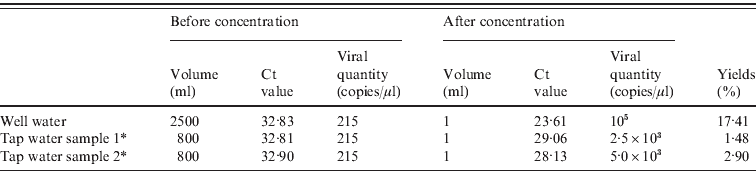
Ct, Cycle threshold.
* The source of the water is from the well; tap water indicates water pumped from the tank into the school water supply on demand.
NoV genotyping results
Segments of the genes encoding NoV RdRp and capsid proteins were amplified and the DNA sequences were analysed from samples collected from two students, two teachers, and well water. The concentration of NoV in positive pumped well-water samples was not enough for sequencing. Comparative sequence analysis showed that all of the NoV gene sequences from humans and water were indistinguishable. Phylogenetic analysis against a collection of reference sequences representing a variety of NoV genotypes showed that the virus responsible for this outbreak was classified as a genotype GII.4 NoV, with the closest identity to GII.4-2006b (AB294793 and EF126966; Figs 3 and 4).

Fig. 3. Phylogenetic tree based on nucleotide sequence of the norovirus RNA-dependent polymerase (nt 4573–4584). The reference strain designation is the Genbank accession number followed by the genotype designation (in parentheses).

Fig. 4. Phylogenetic tree based on the nucleotide sequence of the norovirus capsid gene (nt 5065–5366). The strain designation is the Genbank accession number followed by the genotype designation (in parentheses).
DISCUSSION
We report the results of a large-scale NoV gastroenteritis outbreak in a school located in Guangdong, China caused by consumption of contaminated well water. Several lines of evidence support NoV-contaminated well water as the source of this outbreak. First, we detected NoV in human and well-water specimens, and sequencing of two viral genes indicated these were indistinguishable between the samples. Second, the outbreak was controlled by disinfection of the well. Third, the attack rate was 1·9 times higher in those exposed to well water (untreated) compared to those exposed to municipal (treated) tap water. Finally, students, teachers and staff ate the same foods; no risk factor related to food consumption was identified.
Although GII.4 is the most common cause of NoV infection worldwide [Reference Wu5, Reference Maunula and Von Bonsdorff17], waterborne NoV gastroenteritis outbreaks are normally attributed to the GI genotype [Reference Hewitt9, Reference Lysén10, Reference Parshionikar12], less frequently to genotypes GII.3 and GII.6 [Reference Kim11], and infrequently to GII.4. Maunula et al. [Reference Maunula, Miettinen and von Bonsdorff8] postulated that this may be due to differential stability among unique genotypes. However, no direct evidence has yet been presented that confirms the differences in the frequency of waterborne NoV GII.4 and GI infections are due to differences in NoV genotype stability.
NoV is one of the most important waterborne viruses causing gastroenteritis. There are recent reports of NoV in sewage [Reference Katayama18], river water [Reference Lee and Kim19] and even drinking water [Reference Gutiérrez20]. Often, it is necessary to concentrate the virus in specimens of water because the quantity of virus is small. Membranes (e.g. positively charged [Reference Xiaohong21], negatively charged [Reference Victoria22], and cellulose ester [Reference Zhang23]) are commonly used for this purpose. In this outbreak, the viral copy number in well water and water pumped from the well was 215/μl before concentration and 2·5 × 103–105/μl after concentration. The percentage recovery from well water (17·41%) was much higher than that from the pumped well water [specimen 1 (2·90%) and specimen 2 (1·48%)]. It is likely that the total amount of virus recovered was influenced by differences in the volume of the water samples (2500 ml for the well water as opposed to 800 ml for each of the two pumped well-water specimens). This is supported by a study showing that the initial concentration of virus greatly affects the recovery of virus [Reference Xiaohong21]. Most studies report recovery rates from 3·3% [Reference Victoria22] to 45% [Reference Haramoto24]. In this outbreak, the recovery of virus from cellulose ester membranes was in good agreement with data from similar studies [Reference Xiaohong21, Reference Victoria22].
One limitation of our study is that we were unable to identify the contamination source of the well. An on-site inspection found no specific source of contamination around the well and no evidence of intentional contamination. The well was completely covered and 30 m deep, making direct animal entry virtually impossible. The environment in the immediate vicinity of the well was cluttered with debris, thus it might be possible that leaching from a source contributing to this debris contributed to the contamination.
This is the first report from China in which laboratory studies have been used to confirm the source of a waterborne outbreak of NoV gastroenteritis. Both laboratory and epidemiological evidence indicated that the school's well water was contaminated by NoV GII.4, an uncommon genotype for waterborne NoV outbreaks. Throughout the world, waterborne diseases remain an important cause of morbidity and mortality [Reference Yoder25]. NoV is an important waterborne pathogen in contaminated water [Reference Yoder26]. Provincial authorities should routinely monitor water systems in schools for NoV and repair damaged water systems as necessary. This will reduce morbidity and mortality in children, and allow schools to focus on education.
ACKNOWLEDGEMENTS
This research was financially supported by Guangdong Science and Technology Department (No. 2007A0300007-7). We acknowledge the contributions of Professor Duan Zhaojun and Dr Jin Miao from the Chinese Centre for Disease Control and Prevention for sequencing the capsid gene of the samples. We also acknowledge Professor H. Vennema of the National Institute for Public Health and the Environment of The Netherlands for providing reference viral gene sequences.
DECLARATION OF INTEREST
None.








