Introduction
Community-acquired pneumonia (CAP) is a major life-threatening disease. Mycoplasma pneumoniae infection is one of the most common causes, accounting for 10%–40% [Reference Vervloet1, Reference Jain2]. Unfortunately, M. pneumoniae pneumonia (MPP) has also increased in China recently. One study showed that M. pneumoniae infections made up approximately 70% of all cases of CAP in children over 5 years old [Reference Ma3]. However, there have been no studies with large numbers of paediatric patients with MPP to explore the epidemiology and dynamic changes in the past few years in China. Additionally, most paediatric cases of MPP are benign, but some might develop pleural effusion, multi-organ damage, or serious long-term sequelae, including bronchiolitis obliterans, bronchiectasis or atelectasis [Reference Wang and Duan4]. However, the prevalence of severe MPP (SMPP) in Chinese children is also unclear. Therefore, to better understand the epidemiologic features of MPP in paediatric patients in China, we collected data from a large number of hospitalised patients with MPP in the past 10 years to analyse the distributions of gender, age, seasons and other factors.
Methods
Study subjects
Paediatric patients aged from 1 month to 18 years with pneumonia as the first diagnosis were retrospectively reviewed from June 2006 to June 2016 in Beijing Children's Hospital, Capital Medical University. They were all Chinese living in North China, including North, Northeast and Northwest China. Demographic features of the patients, clinical information and laboratory data were retrospectively collected from the records of all children. All of the paediatric patients were assigned into five age groups: <1 year, 1–3 years, 3–6 years, 6–10 years and 10 years or older. Four distinct seasons were defined as follows: spring (March–May), summer (June–August), autumn (September–November) and winter (December to the following February). This study was reviewed and approved by the Ethics Committee of Beijing Children's Hospital, Capital Medical University (2017-k-35).
Diagnostic criteria
The diagnosis of pneumonia is defined as follows [Reference Hu and Jiang5]: clinical manifestations (fever, cough or wheezing), physical examination and chest imaging with infiltrates. Severe pneumonia defined as pneumonia with one of the followings [6]: (1) poor general condition; (2) increased respiratory rate (infant >70/min, older children >50/min); (3) dyspnoea and cyanosis; (4) multilobe involvement or ⩾2/3 lung involvement; (5) extrapulmonary complication; (6) pleural effusion and (7) transcutaneous oxygen saturation in room air ⩽92%. M. pneumoniae infection is defined as single titres of serum M. pneumoniae antibody ⩾1:320, or seroconversion (increased antibody titres ⩾4 fold) [7]. SMPP is defined as severe pneumonia with M. pneumoniae infection. Exclusion criteria were as follows: subjects with a common cold, mild upper-respiratory tract manifestation with no evidence of pneumonia on chest radiograph or chronic pulmonary disease that might affect the chest X-ray results or aspiration pneumonia or interstitial lung disease.
Detection of M. pneumoniae
M. pneumoniae antibody was detected using the passive agglutination method (Serodia-Myco II, Fujirebio, Japan). The experimental process was strictly in accordance with the instructions. Positive M. pneumoniae infection was defined as single titres of serum M. pneumoniae antibody ⩾1:320, or seroconversion (increased antibody titres ⩾4 fold).
Statistical analysis
All data were analysed using SPSS version 19.0 software. Continuous data are presented as the mean ± standard deviation or range. Continuous variables were analysed using Student's t test, and categorical variables were analysed using the χ 2 test or Fisher's exact test if any expected value was below five. P value <0.05 was considered statistically significant.
Results
Epidemiology of paediatric patients with pneumonia
A total of 27 498 children diagnosed with pneumonia were enrolled in this study; among them, 16 346 were male and 11 152 were female, with a male-to-female ratio of 1.5:1. The mean age was 4.1 ± 3.8 years old, and the median age was 3.2 years, ranging from 1 month to 18 years. The percentage of paediatric patients with pneumonia less than 5 years old was about 66.1% (18171/27498). The distribution of children with pneumonia in different years is shown in Table 1 and Figure 1.
Table 1. Distribution of children with pneumonia in different years

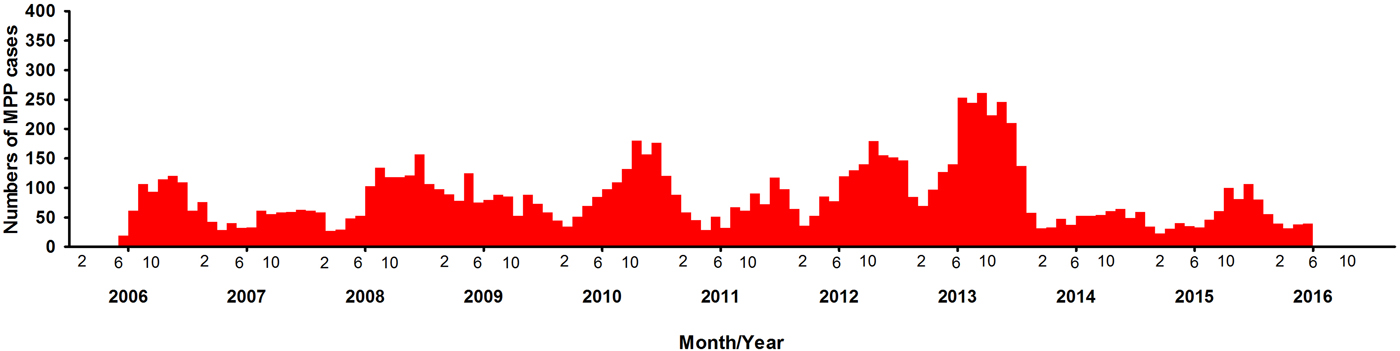
Fig. 1. Distribution of children with MPP in different years.
Demographic features of paediatric patients with M. pneumoniae pneumonia
A total of 10 300 patients were MPP, nearly accounting for 37.5% (10 300/27 498) of all the patients with pneumonia. There were 5415 boys and 4885 girls, with a male-to-female ratio of 1.1:1. There was a significant difference between boys and girls (χ 2 = 322.5, P < 0.0001) (Table 2). During the past 10 years, there have been four outbreaks at the interval of 2–3 years. Over time, the positive rate of MPP has increased in the peak years, reaching to 51.5% from June 2013 to May 2014 (Table 1). The numbers of the patients started to increase at the end of August, with the period of greatest incidence lasting for 5 months every year. This indicated the MPP tended to break out at a particular time, mainly in autumn.
Table 2. Age and seasonal distribution of M. pneumoniae pneumonia in children

Age and season distributions of M. pneumoniae pneumonia
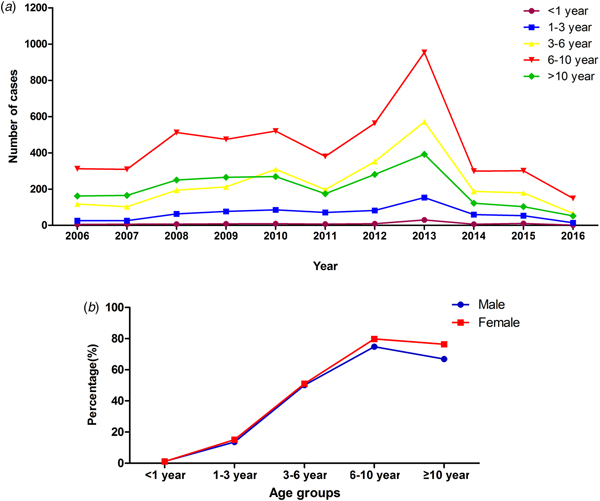
The median age of patients with MPP was 7.4 years (1 month to 18 years). The age group of 6–10 years had the higher rate of MPP than the other age groups (χ 2 = 13 844.14, P < 0.0001), accounting for 76.3% of cases of pneumonia. The peak age did not change during the past 10 years (Fig. 2). However, the rate of MPP in peak year increased with time. Autumn had a higher rate of MPP than the other seasons (χ 2 = 904.9, P < 0.0001) (Table 2).
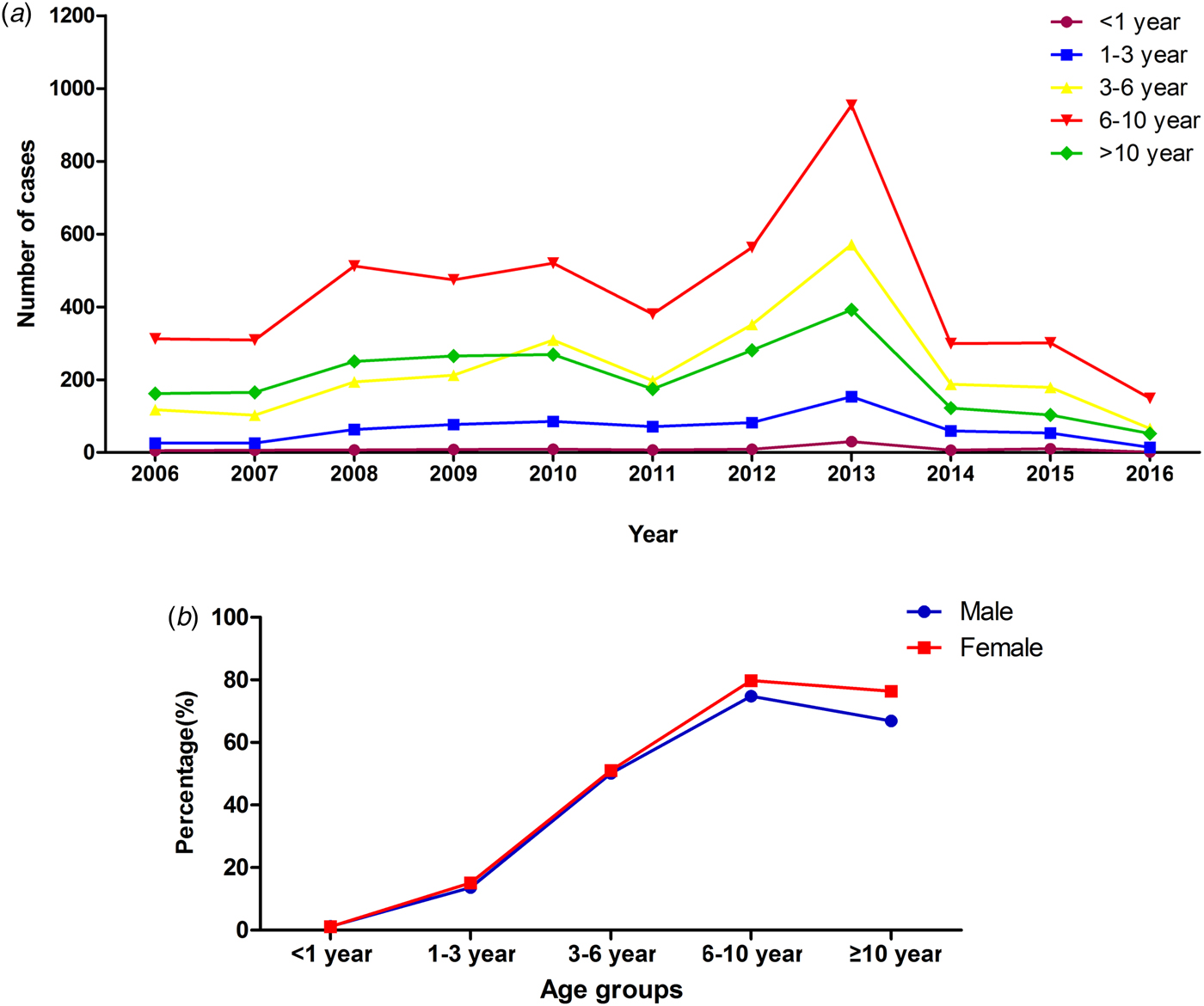
Fig. 2. Distribution of MPP patients with different age in different years and gender.
Comparison of severe M. pneumoniae pneumonia with non-severe M. pneumoniae pneumonia
Approximately 13.0% (1340/10 300) of patients with MPP were SMPP, and the rate of SMPP was increasing year by year, nearly reaching 42.6% in 2016 (Fig. 3). The mean age of paediatric patients with SMPP (6.7 ± 3.0 years old) was younger than that of patients with non-SMPP (7.4 ± 3.2 years old) (t 2 = 3.60, P = 0.0001). The rates of SMPP in patients with MPP in the five age groups (<1 year, 1–3 years, 3–6 years, 6–10 years and more than or equal to 10 years) were 11.2% (11/98), 12.0% (85/709), 15.1% (375/2485), 13.2% (629/4773) and 10.7% (240/2235), respectively. The rate of SMPP was higher in the age group of 3–6 years than the other age groups (χ 2 = 20.74, P = 0.0004). Comparison of the age distribution of patients with MPP showed the peak age of paediatric patients with SMPP to be lower than that of patients with non-SMPP (the peak age was 6 to 10 years old). Autumn was the peak season in the SMPP group, as in the non-SMPP group.

Fig. 3. Prevalence of SMPP patients in different years.
Discussion
M. pneumoniae infection is one of the major pathogens in children with CAP. A study performed in the Suzhou Province in South China reported that the rate of M. pneumoniae infection was ranging from 30.27% to 36.08% [Reference Zhang8, Reference Qian and Wei9]. Our study of north China showed the rate of MPP reached 37.5% in paediatric patients with pneumonia. In addition, we found during the past 10 years, the rate of MPP in the peak years increased, even reaching 51.5% from June 2013 to May 2014. Two to three years will be a peak endemic of MPP in a hospital-based population in North China. Previous studies also showed the same epidemic patterns of MPP in other countries [Reference Jansson, von and Tuuri10]. Eun et al. reported the cyclic occurrence of MPP in Korea, with six epidemic peaks in 18 years separated by 3–4 years [Reference Eun11].
The effect of gender on M. pneumoniae infection is different. In our study, the positive rate of MPP was higher in girls than in boys in North China, as in South China [Reference Xu12]. That may be because girls are more susceptible to M. pneumoniae infection than boys. However, some studies have found that gender is not an important factor for M. pneumoniae infection [Reference Kung and Wang13]. MPP could occur at any age group, especially in pre-school-aged and school-aged children [Reference Nolevaux14]. Defilippi et al. showed the positive rate of M. pneumoniae infection in school-aged children to be 41.76% [Reference Defilippi15]. In Turkey and Japan, the peak age was 7–10 years old [Reference Bosnak16, Reference Ito17]. In Australia, the age group most commonly affected by M. pneumoniae was those of 5–9 years old [Reference Othman, Isaacs and Kesson18]. A study of China demonstrated that children over 7 years old had the highest rate in South China [Reference Xu12]. Most studies report the most common age group of MPP to be over 5 years old, as in our study (6–10 years old). This may be related to human immune response after MP infection. We found the peak age did not change in the past 10 years in North China. However, some studies have reported that most patients with M. pneumoniae infection are younger than 5 years [Reference Huong19].
Paediatric cases of MPP are found year-round, but different studies have reported different results with respect to the seasons [Reference Onozuka, Hashizume and Hagihara20]. In our study, we found autumn to be the peak season in North China, while a study in South China showed the MPP rate to be higher in the summer than in the autumn, spring or winter. A Korean study showed the epidemic peaks of MPP to take place in autumn or winter [Reference Eun11]. One study performed in both children and adults in Zagreb showed MPP to be more common between August and November [Reference Puljiz21]. Two other studies performed in Italy and Australia showed M. pneumonia e infection to be more common in June or July [Reference Defilippi15, Reference Othman, Isaacs and Kesson18]. Foy et al. found that the endemics of M. pneumoniae infection in Seattle have no significant seasonal fluctuations [Reference Foy22]. Another study conducted between 1986 and 2004 in Korea showed that the peak season changed over time [Reference Eun11]. Before 1996, the peak epidemics of M. pneumoniae infection took place in summer, while the later epidemics took place in autumn or early winter.
In recent years in China, more and more cases of SMPP have been reported [Reference Taylor and Blackbourn23]. However, no large case studies have been performed to investigate the prevalence of SMPP. Our study showed that SMPP made up about 13.0%, of all cases, with that proportion increasing over time. The increased positive rate of SMPP in China might be related to the high rate of macrolide-resistance of M. pneumoniae (more than 90%) [Reference Liu24, Reference Feng25]. There was no difference in seasons between SMPP and non-SMPP paediatric patients. The mean age of paediatric patients with SMPP was younger than that of patients with non-SMPP in our study. This suggested that younger paediatric patients are susceptible to SMPP. Therefore, in clinical settings, paediatricians should pay more attention to younger patients with MPP.
Conclusions
MPP and SMPP are more and more common in North China, especially in children from 6 to 10 years old. Paediatric patients with SMPP tend to be younger than those with non-SMPP. MPP outbreaks occur every 2–3 years in North China. Autumn is the peak season, unlike in South China. Understanding the epidemiological characteristics of paediatric MPP can contribute to timely treatment and diagnosis, and may improve the prognosis of children with SMPP.
Data
The datasets collected and analysed during the current study are available from the corresponding author upon reasonable request.
Author ORCIDs
Li-Wei Gao, 0000-0001-7652-7456
Acknowledgements
We would like to thank all the people involved in our study.
Author contributions
All of the authors had access to the full dataset (including the statistical reports and tables) and take responsibility for the integrity and the accuracy of the data analysis. BPX, KLS and LWG conceived the study. JY, YHH, XYL, XLF, JXH, JL and YG collected the data and designed the analysis. LWG interpreted the data. LWG wrote the first draft of the manuscript. BPX reviewed and approved the final report. All authors read and approved the final manuscript.
Financial support
All phases of this study were supported by Capital's Funds for Health Improvement and Research (2014-1-2094) and Capital Characteristic Clinic Project (Z171100001017081).
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
This study was reviewed and approved by the Ethics Committee of Beijing Children's Hospital Affiliated with Capital Medical University (2017-k-35). Informed consent was deemed unnecessary due to the retrospective collection of data. Identification of the patients was kept confidential.