INTRODUCTION
Legionellosis, an infection caused by the intracellular bacterial pathogen, Legionella (most commonly L. pneumophila), is associated with the distinct clinical illnesses: Pontiac fever, a self-limited flu-like illness, and Legionnaires' disease (LD). LD, which presents as severe pneumonia, results in 8000–18 000 hospitalizations for US residents annually with a case-fatality rate of ∼8% [Reference Marston1, Reference Ricketts and Joseph2]. Most of what we know about legionellosis has been learned from investigation of outbreaks, but over 80% of cases occur sporadically [Reference Marston, Lipman and Breiman3, Reference Fields, Benson and Besser4]. Host characteristics such as older age, male sex, cigarette smoking and underlying lung disease increase the risk for sporadic cases of the disease; however, the environmental factors related to legionellosis have been much more difficult to identify [Reference Storch5, Reference England6].
Legionella bacteria are ubiquitous in natural and man-made aquatic environments and multiply inside free-living amoebae in the presence of warm water (25–42°C) [Reference Katz and Hammel7, Reference Fields8]. The interaction occurs in building water systems and is enhanced by the presence of scale, sediment, and stagnation. Humans acquire infection through inhalation of water aerosols such as those generated by whirlpool spas or cooling towers [Reference Den Boer9–Reference Thomas, Mundy and Tucker17]; aspiration of contaminated water from potable water sources has also been implicated as a source of transmission [Reference Yu18].
In the spring of 2003, five states in the Mid-Atlantic region of the United States recognized an increase in the reported number of sporadic, community-acquired LD cases. From January 2003 to August 2003, the Centers for Disease Control and Prevention (CDC) received 374 reports of legionellosis in these five states compared to an average of 161 (range 107–225; CDC, unpublished data) during the same months in the previous 13 years. This increase seemed to coincide with some of the heaviest precipitation on record in the last century in this geographical region. We initiated an investigation to determine if legionellosis rates were indeed above normal and if the increase in reported illness was localized in the Mid-Atlantic states. We also wanted to identify explanations for the increase. To evaluate the impact of rainfall and temperature on incidence of sporadic legionellosis, we conducted an ecological study examining the relationship between climatic data in several Mid-Atlantic states to the corresponding legionellosis disease data.
METHODS
Case ascertainment
Legionellosis is a nationally notifiable disease in the United States. Legionellosis cases are reported to the CDC by state health departments via a passive, electronic reporting system: the National Electronic Telecommunications System for Surveillance (NETSS). A case of legionellosis is defined as a clinically compatible illness with laboratory confirmation of infection. Laboratory criteria for diagnosis include any one of the following: isolation of Legionella from respiratory secretions, lung tissue, pleural fluid or other normally sterile fluid; demonstration of a fourfold or greater rise in reciprocal immunofluoresence antibody titre to ⩾128 against L. pneumophila serogroup 1 between paired acute- and convalescent-phase serum specimens; detection of L. pneumophila in respiratory secretions, lung tissue, or pleural fluid by direct fluorescent antibody testing; or detection of L. pneumophila serogroup 1 antigens in urine by radioimmunoassay or enzyme-linked immunosorbent assay.
Aggregated legionellosis case counts by month of onset were obtained from NETSS for January 1990 to December 2003. The corresponding annual population estimates for Delaware, Maryland, North Carolina, Pennsylvania, and Virginia (Mid-Atlantic states) were obtained from the U.S. Census Bureau. These states were included in the analysis because public health officials from these states contacted the Respiratory Diseases Branch at CDC to express concern regarding an increase in reported legionellosis cases. In addition, it was evident from reviews of NETSS data that these states were experiencing much higher rates of legionellosis than others. Aggregated monthly legionellosis incidence was calculated using total monthly case counts for these states and the corresponding total population.
We reviewed state surveillance methods and legionellosis case-patient data to determine if there were any significant changes that would have led to increased case ascertainment. A data abstraction tool was used to collect additional information on case-patients including demographics, past medical history, recent travel history, possible exposures, and clinical data. All case-patients included (n=112) were from the Mid-Atlantic states with onset dates between 5 May and 20 July 2003. These case-patients were selected because their illness occurred during the initial months when the increase in legionellosis was recognized. Those selected had contact information listed on the surveillance form and were able to be contacted by telephone. Case-patient records were reviewed to identify any evidence of clustering of cases in the same town or city or common community or travel exposures. In addition, we administered a hypothesis-generating questionnaire to a convenience sample of 19 of the 112 case-patients to explore water exposures in more detail. This interview included questions about presence or absence of in-home flooding, type of water heater and water supply, changes in water supply or plumbing repairs, and potential domestic or occupational exposures. Case-patients were also asked about exposures to large buildings or factories (presence of cooling towers) and if there had been any recent construction, plumbing repairs, or flooding in their place of work.
To explore alternative explanations for the increase in legionellosis, we collected data on urine antigen testing, the test used to diagnose the vast majority of cases. Due to emergence of Severe Acute Respiratory Syndrome (SARS) in early 2003, we were concerned that increased vigilance among clinicians may have contributed to an increase in pathogen testing and thus an increase in legionellosis case ascertainment. Most cases were diagnosed by Legionella urinary antigen tests and during the increase ∼60% of these tests were performed by commercial laboratories. Therefore, we evaluated data collected from leading commercial laboratories in the United States to assess if there was a change in urine antigen testing volume in the Mid-Atlantic states. We compared the volume of urine antigen testing in 2002 to the volume in 2003. In addition, we explored whether there had been any significant changes in the formulation of the urine antigen tests used in the United States that might have led to increased sensitivity or decreased specificity.
Rainfall analyses
To identify whether rainfall and temperature correlated with legionellosis, monthly total rainfall and average temperatures by state were obtained for 1998–2003 from the National Oceanic and Atmospheric Administration's National Climatic Data Center [19]. The average monthly temperatures for each state are based upon continuous temperature measurements taken at weather stations at several locations across each state. Temperature was included in the analysis to account for changes in Legionella growth that occurred as a result of warm temperatures. Monthly incidence and the corresponding rainfall and temperature data were used to calculate Pearson's correlation coefficient (r). Estimates of mean rainfall and temperature for each state were computed using a weighted average based on each state's population. We evaluated the correlation between LD and rainfall in concurrent months. In a second analysis we applied a 1-month lag to the temperature and rainfall data behind monthly legionellosis case counts to account for the time required for Legionella amplification and disease manifestation.
To further evaluate a possible association between rainfall and legionellosis, a negative binomial regression model was used to determine if an increase in rainfall was associated with an increased risk for legionellosis. Monthly incidence of legionellosis was the outcome variable and monthly rainfall was the primary independent variable of interest. The model included the variables state and year to control for potential confounding. Temperature was added as a dependent variable and a product term of rainfall and temperature was included to evaluate for statistical interaction. Estimates obtained from the regression model were used to calculate the risk ratio (RR) for legionellosis based upon rainfall amount. The parameter estimate from the regression model was used to estimate the risk of legionellosis associated with increasing rainfall amounts.
All analyses were conducted using SAS version 9 (SAS for Windows; SAS Institute, Cary, NC, USA). We calculated 95% confidence intervals (CI) for all risk ratios and two-tailed P values; P<0·05 was considered statistically significant.
RESULTS
Increase in cases confirmed
Legionellosis case counts in the Mid-Atlantic states increased dramatically above the normal monthly average beginning in May 2003 (Fig. 1). Between May and September 2003 the rate of legionellosis was 315% higher than the average rate during the same months in the 13 previous years (1·23 cases/100 000 in 2003 compared to 0·39 cases/100 000 in 1990–2002; P<0·001). Public health officials in the Mid-Atlantic states did not identify any changes in legionellosis surveillance which would have resulted in increased case ascertainment or reporting.
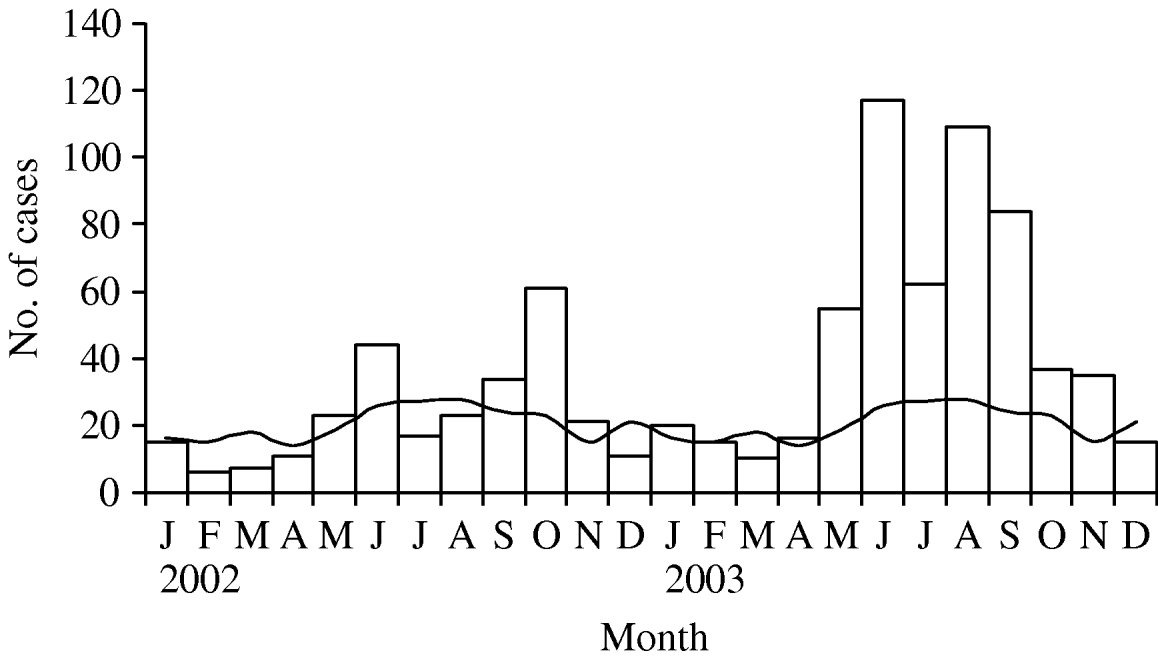
Fig. 1. Legionellosis cases per month in the Mid-Atlantic states (Delaware, Maryland, North Carolina, Pennsylvania, Virginia) in 2002 and 2003 (□), compared to the 1990–2001 monthly average (—).
Of 234 case-patients in the Mid-Atlantic states from May 2003 to July 2003, 112 (48%) were contacted to collect additional information. Case-patients were an average of 59 years of age (range 21–91 years), and 47 (42%) had a history of smoking or underlying lung disease. An additional 42 (38%) case-patients had a chronic immunocompromising condition such as HIV, cancer on chemotherapy, chronic renal failure, lupus, or chronic steroid use. These case-patients did not report any common travel destinations or point-source exposures with the exception of two case-patients who worked in the same hospital. This suggested that the cases were sporadic and not part of a point-source outbreak. Twenty-five (22%) case-patients reported local travel within their state or a neighbouring state. No case-patients reported extensive domestic travel, international or cruise-ship travel during the 2 weeks prior to the onset of symptoms. Two (2%) case-patients reported a hospital stay during the 2 weeks prior to illness onset. One hundred and five (94%) case-patients were hospitalized and 11 (10%) died. One hundred and six (95%) of case-patients were diagnosed using the Legionella urinary antigen test. Legionella was isolated on culture for only three (3%) case-patients. Environmental isolates were not available for comparison as the cases were reported via routine passive surveillance and were not a part of an outbreak investigation.
Among 19 case-patients who answered additional hypothesis-generating questions regarding occupational and domestic water exposures, only one (5%) reported recent flooding in his home and one (5%) described recent plumbing repairs. None of the case-patients had travelled recently or were aware of any exposure to traditional sources of infection, such as whirlpool tubs or cooling towers. Municipal water supplied 17 (89%) case-patient homes and two (11%) case-patient homes were served by private well water.
During the period that the increase in cases occurred, there was no apparent increase in urine antigen testing compared to the previous year to explain the increase in cases. From May to July 2002 two commercial laboratories received 1090 requests for urine antigen testing in the Mid-Atlantic states compared to 993 during the same period in 2003. In addition, test manufacturers, Binax Inc. (Scarborough, ME, USA) and Wampole Laboratories (Princeton, NJ, USA), of the two most widely used commercial urinary antigen tests in the United States reported that there had not been significant changes to the tests that would have led to changes in sensitivity or specificity. Additionally, sales of test kits did not change markedly during the period that the increase in cases occurred.
A review of trends in case reports of nationally notifiable diseases during the time of the increase in legionellosis did not reveal any marked increases in reports of other diseases to suggest an increase in disease reporting by physicians, infection control staff, or health departments. Additionally, there were no marked changes in state or national surveillance methods during this period.
Rainfall analyses
Monthly rainfall averaged 15·7 cm from May 2003 to September 2003 in the Mid-Atlantic states, well above the normal monthly average of 10·4 cm in the same months during the previous 13 years. Both rainfall and legionellosis rates were highly variable between 1998 and 2003, but increases in rainfall generally corresponded to increased legionellosis rates (Fig. 2). Pearson's r was 0·40 for the correlation between mean rainfall and legionellosis and 0·39 for legionellosis and mean temperature (both P<0·001). The correlations for monthly case counts of legionellosis and rainfall (r=0·24, P=0·002) and temperature (r=0·25, P=0·001) in the preceding calendar month were lower than rainfall and temperature in the concurrent month, but still statistically significant.
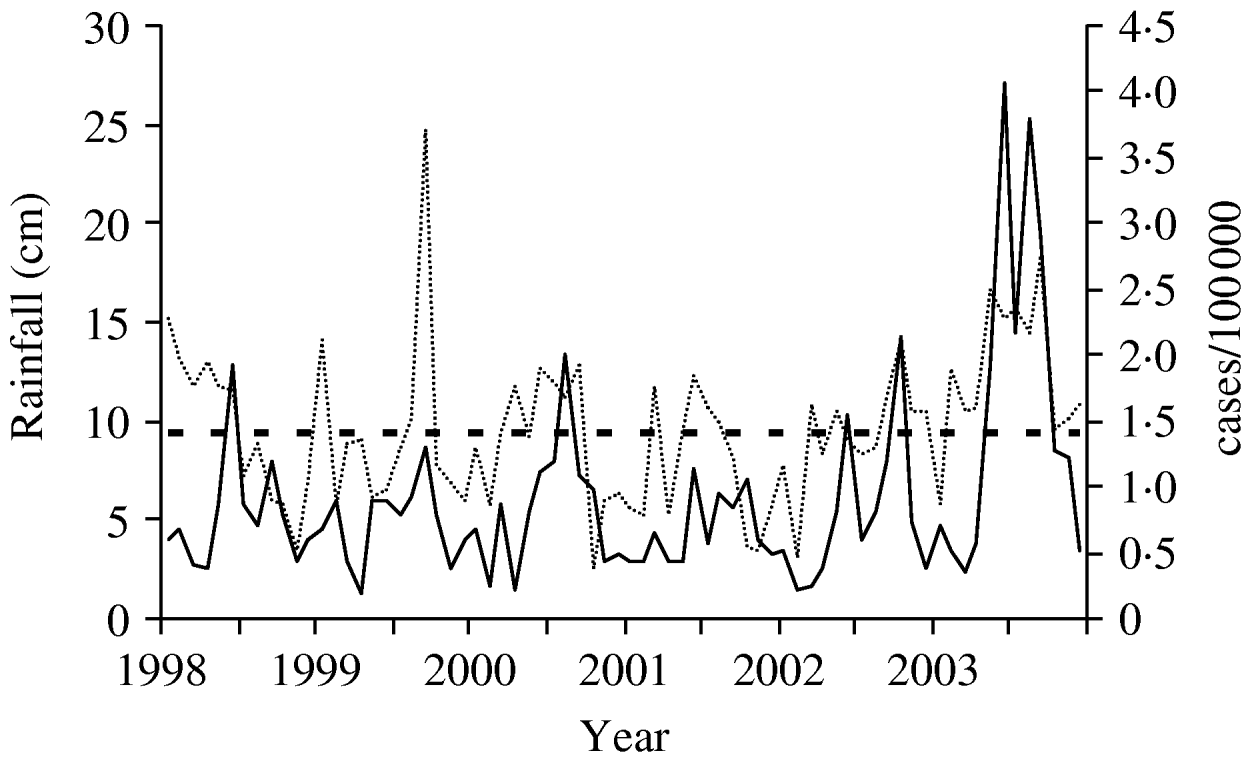
Fig. 2. Legionellosis rate and amount of rainfall over time, Mid-Atlantic states, 1998–2003. ——, Legionellosis rate; · · · · · ·, mean monthly rainfall; - - - -, mean rainfall 1990–2002 (cm).
The legionellosis and rainfall relationship was explored further with a negative binomial regression analysis. Rainfall and temperature were included as continuous covariate variables while controlling for the categorical variables state, year, and month. In this model temperature was not significant. However, temperature and month are highly correlated. Therefore, when month was removed from the model temperature was highly significant (P<0·0001). The final model included temperature and rainfall while controlling for state and year. There was no evidence of an interaction between rainfall and temperature. The model confirmed that rainfall and temperature were independently associated with increased legionellosis. An increase of 1·0 cm in rainfall was associated with a 2·6% increased risk for legionellosis (RR 1·026, 95% CI 1·012–1·040). A degree increase in Celsius was associated with a 2·8% increased risk for legionellosis (RR 1·028, 95% CI 1·021–1·035).
The risk of legionellosis increased in a multiplicative relationship with increases in rainfall as predicted by our model (Fig. 3). The average monthly rainfall for 1990–2002 from May to September for the Mid-Atlantic states was 10·4 cm compared to 15·7 cm from May to September 2003. The model predicted that this change in rainfall corresponds to an increase in risk of LD of ∼14·6% (RR 1·146, 95% CI 1·067–1·231).
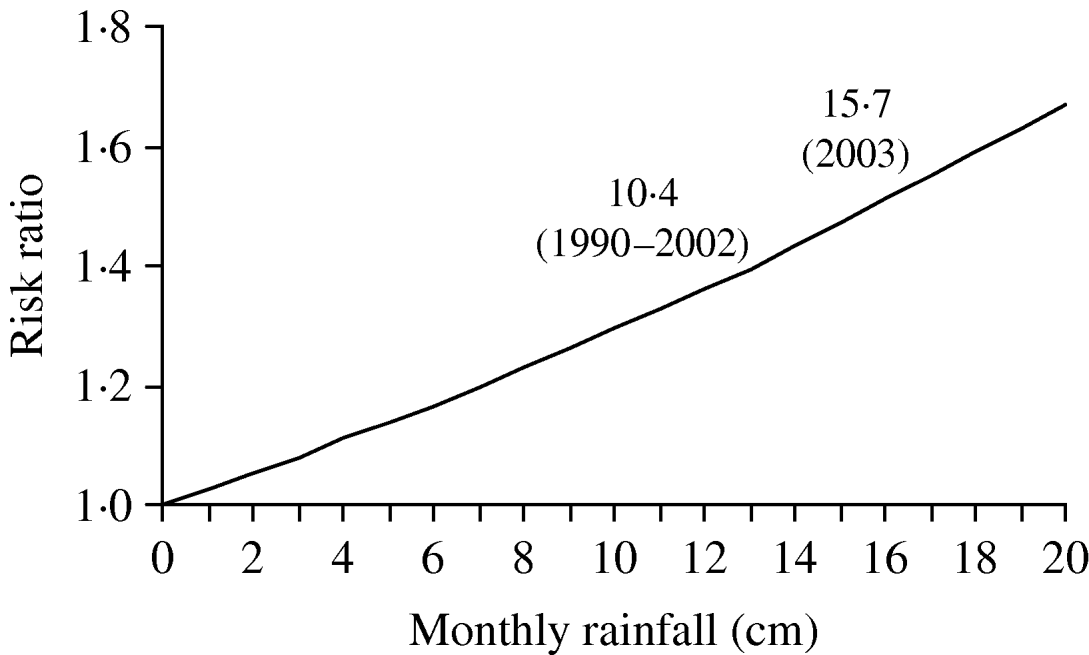
Fig. 3. Risk of legionellosis with increased rainfall as predicted by Poisson regression model, controlling for state and year, 1999–2003.
DISCUSSION
The first recognized outbreak of LD in 1976 and several subsequent epidemiological outbreak investigations have elucidated many individual and environmental risk factors for legionellosis. However, for the 80% of legionellosis cases which are sporadic, the environmental risk factors for acquiring sporadic legionellosis are poorly defined [Reference Marston, Lipman and Breiman3]. One case-control study of sporadic community-acquired LD in the United States revealed that LD case-patients were more likely than controls to have resided near a construction site or to have worked in construction [Reference Storch5]. During May to September, 2003 rainfall in the Mid-Atlantic states was 54% higher than the historical average during these same months. Legionellosis rates were also above historical limits for these same months. There were no appreciable changes in surveillance, case ascertainment, or urine antigen testing to explain the increase. In the analysis of rainfall amounts and legionellosis rates over a 14-year period, we found that rainfall in the concurrent month correlated significantly with legionellosis rates, and multivariable analyses supported the hypothesis that increased rainfall is independently associated with increased risk for legionellosis. Episodes of heavy rainfall have been described anecdotally in a few early legionellosis outbreak reports [Reference Glick20–Reference Kool22]. And a study of legionellosis cases in the greater Philadelphia area from 1995 to 2003 described a dose–response relationship for precipitation and humidity and the occurrence of legionellosis cases [Reference Fisman23].
Increased mean monthly temperature was also independently associated with increased risk for legionellosis. This is consistent with the microbial ecology of the organism; Legionella growth is enhanced by warm temperatures [Reference Fields, Benson and Besser4]. This finding is also reinforced by another epidemiological study that showed that legionellosis rates are consistently highest during the warm summer months [Reference Marston, Lipman and Breiman3]. The role of warm temperatures in Legionella growth may explain why increases in rainfall in cooler months were not always accompanied by large increases in the rate of legionellosis.
It is important to recognize that this was an ecological study, so there may have been confounding variables which we were unable to measure or did not consider. Therefore, caution should be taken in assigning causality to these findings. Other factors could contribute to the increase in legionellosis cases and may explain why the actual number of reported cases of legionellosis from May to September 2003 exceeded the increase in the number of cases that our model predicted based upon increased rainfall amount. For example, the completeness of reporting of legionellosis cases may have increased somewhat in the wake of the SARS outbreak, a possibility we were not able to measure. In addition, while there was no indication that the increase in legionellosis incidence was due to travel or common-source outbreaks, the conclusion that there was an increase in sporadic disease may be subject to self-reporting bias.
Although Legionella is a waterborne pathogen, it is unclear exactly how rainfall would lead to increased incidence of legionellosis. Several different mechanisms may contribute to the increase in risk. One hypothesis was that heavy rains might have caused flooding in the case-patients' homes or immediate environment, which predisposed them to legionellosis. Flooding was identified as a precipitant of one outbreak in a bar in St Louis, where a sump pump in a flooded basement promoted Legionella amplification and subsequent aerosolization [Reference Kool22]. However, in the hypothesis-generating interviews in our study, only one (5%) of 19 case-patients reported flooding at his home in the 2 weeks prior to his illness onset. One study of sporadic LD found that having received water from a non-municipal water supply (e.g. private well) [Reference Straus24] was associated with increased risk for disease. We hypothesized that the heavy rains might have led to contamination of untreated groundwater, however, only two (11%) case-patients reported use or consumption of untreated water.
Other studies have examined the influence of rainfall on infectious diseases. Increased incidence of melioidosis, another bacterial respiratory illness endemic to Southeast Asia and Northern Australia, has been linked to preceding heavy rainfall [Reference Dance25, Reference Currie and Jacups26]. Melioidosis is the most common cause for fatal community-acquired pneumonia in the northern portion of Australia's Northern territory. Like Legionella, the organism which causes melioidosis, Burkholderia pseudomallei, is an environmental bacterium of soil and water. Inhalation of the bacteria is a well-recognized mode of disease transmission, and melioidosis incidence correlates positively with total rainfall, although the reason for this association is unknown [Reference Supputtamongkal27, Reference Currie28].
A relationship between rainfall and acute gastroenteritis has also been shown. Using a complete database of all waterborne gastrointestinal disease outbreaks from 1948 to 1994, Curriero et al. examined the relationship between rainfall and waterborne disease outbreaks [Reference Curriero29]. Of 548 waterborne outbreaks, mostly gastroenteritis, which occurred between 1948 and 1994 in the United States, 51% were significantly associated with preceding extreme precipitation above the 90th percentile (P=0·002), and 68% were preceded by precipitation events above the 80th percentile (P=0·001). The majority of the outbreaks were attributed to changes in quality of the potable water supply; 133 (24%) waterborne outbreaks were known to be from surface water contamination and 197 (36%) from groundwater contamination.
Studies that have focused specifically on water quality parameters and microbial growth have shown that many bacteria and protozoa increase in water samples after rain [Reference Atherholt30, Reference Geldreich31]. This increase can be attributed to increased particulate matter, i.e. turbidity of the water supply. An important component for Legionella amplification, in addition to a warm, aquatic environment, is the presence of free-living protozoa [Reference Fields, Benson and Besser4, Reference Fields8, Reference Rowbotham32]. Legionella survive as intracellular parasites of protozoa, so theoretically increased protozoa in the water supply would increase the opportunity for Legionella growth. Whether deficiencies in the treatment of potable water supplies must occur concurrently with heavy rains to result in an increase in Legionella in the water supply remains unclear. If Legionella were to increase in potable water supplies after heavy rainfall, it is plausible that mechanisms of acquisition in the home, such as aspiration or inhalation of aerosols, would be more likely to lead to exposure and disease [Reference Yu18].
Our evidence suggests that heavy rainfall is associated with increased risk for legionellosis. Public health officials and clinicians should be aware of the increased risk for legionellosis during periods of heavy rainfall, as knowledge of this risk may influence diagnostic and treatment decisions surrounding cases of community-acquired pneumonia. Environmental studies designed to evaluate the precise effect of rainfall on Legionella growth, spread, and acquisition would be useful to explain our observed association. Identification of the specific mechanisms and events that lead to increased transmission might lead to opportunities for prevention of sporadic legionellosis.
ACKNOWLEDGEMENTS
We are indebted to Karen Cowgill, Brendan Flannery, Matthew Moore, Joseph Carpenter, and Jacob Kool (Centers for Disease Control and Prevention), Claire Newbern (Philadelphia Department of Public Health), Andre Weltman (Pennsylvania Department of Health), Eileen Koski (Quest Diagnostics), Linda Kaimins (ARUP laboratories), Anne Jepsum (Binax, Inc.), and Wampole Laboratories for their contributions to this study. This study received financial support from the Centers for Disease Control and Prevention, Atlanta, GA, USA.
DECLARATION OF INTEREST
None.





