INTRODUCTION
Prostate cancer (PC) is the most commonly diagnosed cancer in men in the USA [Reference Horner1]. Emerging evidence has suggested that infection within the prostate stimulates the immune system to recruit more cytokines and accumulate reactive oxygen species, which have been identified as being associated with increased cellular proliferation and carcinogenesis [Reference De Marzo2, Reference Coussens and Werb3]. Many studies have indicated that bacterial and chronic prostatitis are associated with an increased risk of PC [Reference Dennis, Lynch and Torner4–Reference Roberts6]. Some studies have also suggested that sexually transmitted infection (STIs) may play an important role in the development of PC [Reference Taylor, Mainous and Wells7–Reference Rosenblatt, Wicklund and Stanford11]. In particular, one study reported a 15% reduction in the relative risk of PC in men circumcised before their first sexual intercourse since circumcision could reduce risk for acquiring STIs [Reference Wright, Lin and Stanford12]. However, this claim has been refuted by several studies which failed to observe a significant association between STI and PC [Reference Huang13–Reference Dennis15].
Therefore, the relationship between STIs and PC remains inconclusive to date. In addition, all previous studies investigating this association used survey study or case-control study designs, and therefore were unable to comment on the possibility of a causal relationship between STIs and PC. Furthermore, while it has been indicated that the degree of relationship between STIs and PC varies according to ethnicity [Reference Huang13, Reference Cheng, Witte and Jacobsen14], no previous investigation has been conducted outside of Western society. Therefore, this study aimed to investigate the risk of PC following STI by using a population-based matched-cohort design in Taiwan. In Taiwan, over 98% of residents are of Han Chinese ethnicity and the homogenous population may exempt our study from potential confounding by race.
METHODS
Database
The data utilized in this study were sourced from the Longitudinal Health Insurance database (LHID2000). Taiwan began its National Health Insurance (NHI) programme in 1995, with 22·60 million of Taiwan's 22·96 million population being enrolled in this programme in 2007. The LHID2000 contains original medical claims data and registry files for 1 000 000 individuals randomly sampled from all enrollees in the NHI programme in 2000. This database was created by the Taiwan National Health Research Institute, which also validated that there was no significant difference in gender distribution between the patients in the LHID2000 and all the enrollees of the NHI. Previous studies have also demonstrated the high validity of the data from the NHI programme [Reference Cheng16–Reference Chung and Kang18] with hundreds of studies employing data from the Taiwan NHI having been published in internationally peer-reviewed journals [Reference Chen19, Reference Chen20].
This study was exempt from full review by the Institutional Review Board since the LHID2000 consists of anonymized secondary data released to the public for research purposes.
Study population
This retrospective matched-cohort study utilized a study group and a comparison group to examine the relationship between STIs and the subsequent risk of PC. The study group was selected by first identifying the 1894 male patients who had visited outpatient care centres and had received their initial diagnosis of a STI between 1 January 2001 and 31 December 2004. In this study, the STIs included genital herpes [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 054.1, 054.10, 054.13, 054.19], genital warts (ICD-9-CM code 078.11), Chlamydia (ICD-9-CM codes 078.88, 079.88, 079.98, 099.41, 099.53-099.55), gonorrhoea (ICD-9-CM codes 098.0, 098.1, 08.10-098.19, 098.2, 098.30-098.34), syphilis (ICD-9-CM codes 091.0, 095.4, 095.8), and epididymitis/orchitis (ICD-9-CM codes 604, 604.0, 604.9, 08.13, 098.33). The patient's first visit for a STI between 1 January 2001 and 31 December 2004 was assigned as their index date. In order to increase the diagnostic validity of STIs in this study, we only included STI subjects who had received at least two STI diagnoses during the period between 2001 and 2004 separated by a maximum of 1 month. In Taiwan, the first diagnosis of a condition reflects the clinician's utilization of a diagnostic test to assess the condition in question, while the second diagnosis indicates the presence of the condition based on the outcome of both the diagnostic test and a clinical examination. Therefore, by limiting our STI subjects to those who received at least two STI diagnoses, we ensured that all of our STI subjects received a positive diagnosis based on their symptoms, medical history, and the results of at least one diagnostic test.
We excluded patients aged <40 year old (n = 826) since PC is a rare occurrence in this age group. We further excluded all patients who had ever received a diagnosis of PC (ICD-9-CM code 185, malignant neoplasm of prostate) prior to their index date (n = 13). However, since the NHI was initiated in 1995, patients who had received a diagnosis of PC before 1996 but had not been diagnosed with a subsequent PC would have been included. In total, 1055 male subjects with a STI were included in the study group.
We also retrieved the comparison cohort from the LHID2000. A ratio of 1:10 was used to randomly extract 10 550 comparison subjects from LHID2000, matched with the study group based on age group (40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, >74 years) and the year of index date. We selected 10 comparison subjects per study subject on account of the low prevalence of PC. This large sample size allowed us to detect the real difference in the incidence of PC between study cohort and comparison cohort. For the comparison cohort, we assigned their first ambulatory care visit occurring in the index year as the index date. We ensured that none of the selected comparison patients had ever received a diagnosis of STI since the initiation of the Taiwan NHI programme. All the selected comparison subjects were similarly ensured to have never received a PC diagnosis between the initiation of NHI and their index date.
As a result, 1055 study subjects and 10 550 comparison subjects were included in this study. Each subject (n = 11 605) was individually tracked for a 5-year period beginning with their index date (study period was from 2001 to 2009). Subjects who received a diagnosis of PC during the follow-up period were identified. We also censored subjects who died during the 5-year follow-up period. Overall 1015 subjects had died during the study period; 118 (11·2%) were from the study cohort and 897 (8·5%) were from the comparison cohort.
Statistical analysis
SAS System for Windows, version 8.2 (SAS Institute Inc., USA) was used to perform all the statistical analyses conducted in this study. We used χ 2 tests to examine differences in the distribution of sociodemographic characteristics including the urbanization level of each subject's residence (five levels: 1 = most urbanized, 5 = least urbanized), geographical region (Northern, Central, Eastern, Southern Taiwan) and monthly income (0, NT$1–15 840, NT$15 841–25 000, ⩾NT$25 001) between patients diagnosed with a STI and those who did not receive such a diagnosis. We also compared the prevalence of selected medical comorbidities between the study and comparison cohorts. The medical comorbidities consisted of hypertension, diabetes, hyperlipidaemia, obesity, chronic prostatitis, history of vasectomy, tobacco use disorder, and alcohol abuse/alcohol dependency syndrome and were included on account of having been reported to be the potential risk factors for PC. In this study, we only included these comorbidities if they occurred in an inpatient setting or if they appeared in two or more ambulatory care claims coded within 1 year prior to the index date.
We performed a Kaplan–Meier survival analysis and a log-rank test to examine the differences in the risk of PC between the study and comparison cohorts. We calculated incidence rates and their corresponding 95% Wald confidence interval (95% CI) of PC during the 5-year follow-up period. Stratified Cox proportional hazards regressions (stratified on age group and index year) were further performed to compute the hazard ratio (HR) of PC during the 5-year follow-up period after adjusting for the above-mentioned variables. We also found that the proportional hazards assumption was satisfied as the survival curves for both study and comparison cohorts had survival functions that were approximately parallel to each other. A value of P < 0·05 was considered to be statistically significant.
RESULTS
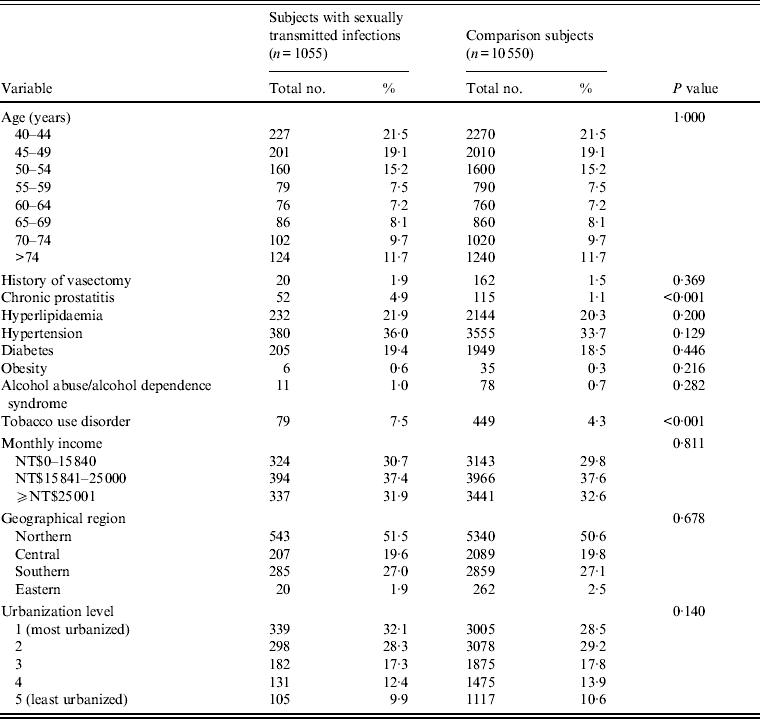
The mean age for the 11 605 sampled subjects was 56·5 (± s.d. 13·0) years. Table 1 displays the distributions of demographic characteristics as well as medical comorbidities according to the presence/absence of STI after matching for age group and index year. There was no significant difference in the distribution of urbanization level, geographical region, or monthly income between the study and comparison cohorts. In addition, no significant differences were observed in the prevalence of hypertension, diabetes, hyperlipidaemia, obesity, history of vasectomy, and alcohol abuse/alcohol dependency syndrome between patients with and without a STI. However, subjects with a STI had a higher prevalence of chronic prostatitis (4·9% vs. 1·1%, P < 0·001) and tobacco use disorder (7·5% vs. 4·3%, P < 0·001) than subjects without a STI.
Table 1. Distributions of demographic characteristics and comorbid medical disorders for patients in Taiwan with sexually transmitted infections and comparison subjects, 2001–2004 (n = 11 605)

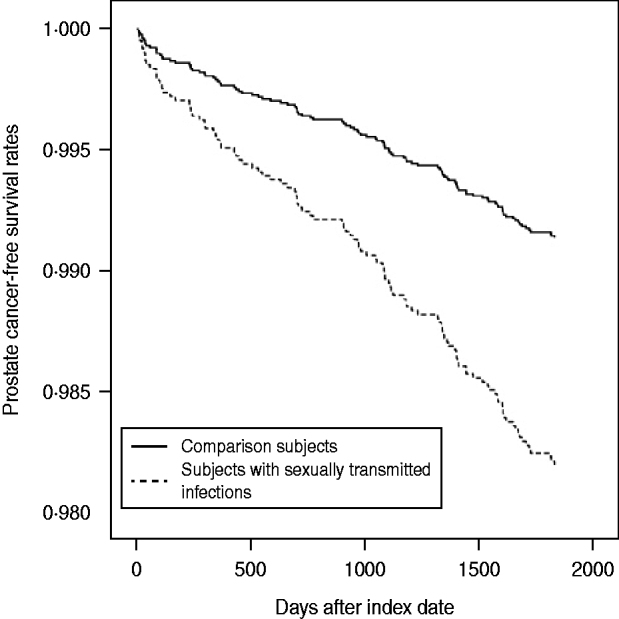
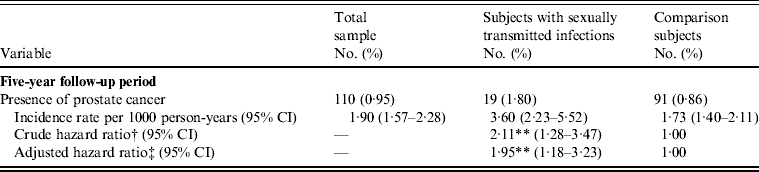
The incidence rate of PC during the 5-year follow-up period is presented in Table 2. Of the 11 605 subjects, 110 (0·95%) had received a diagnosis of PC during the 5-year follow-up period; 19 (1·80%) were from the study cohort and 91 (0·86%) were from the comparison cohort. The incidence rate was 3·60 (95% CI 2·23–5·52) and 1·73 (95% CI 1·40–2·11) per 1000 person-years for the study and comparison cohorts, respectively. Furthermore, log-rank tests revealed that patients with a STI had significantly lower PC-free survival rates than comparison subjects (P < 0·001). The Kaplan–Meier method PC-free survival curves for subjects with and without STIs are presented in Figure 1.

Fig. 1. Prostate cancer-free survival rates for subjects with sexually transmitted infections and comparison subjects.
Table 2. Incidence rate and hazard ratios for prostate cancer in sample patients during the 5-year follow-up period starting from the index date (n = 11 605)

CI, Confidence interval.
† Hazard ratio was calculated by the stratified Cox regression model (stratified on age group).
‡ Adjustments are made for patients' geographical location, urbanization level, monthly income, history of vasectomy, chronic prostatitis, hypertension, diabetes, hyperlipidaemia, obesity, tobacco use disorder, and alcohol abuse/alcohol dependence syndrome.
** P < 0·01.
Table 2 also presents the HRs for PC within the 5-year follow-up periods after the index date. After adjusting for urbanization level, geographical region, monthly income, hypertension, diabetes, hyperlipidaemia, obesity, chronic prostatitis, history of vasectomy, tobacco use disorder, and alcohol abuse/alcohol dependency syndrome and censoring the individuals that died during the follow-up period, a stratified Cox proportional hazards regression (stratified on age group and index year) suggested that the HR for PC within the 5-year period for subjects with a STI was 1·95 (95% CI 1·18–3·23) that of comparison subjects.
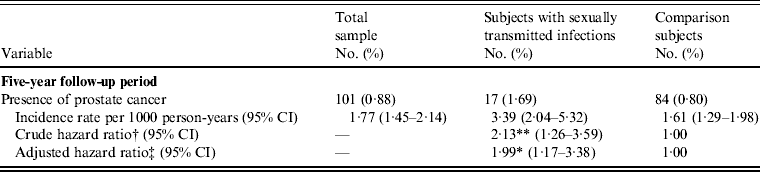
In order to isolate the potential confounding effect of chronic prostatitis on the relationship between STIs and PC, we further analysed the HRs for PC after excluding those subjects with a history of chronic prostatitis. Table 3 shows that after excluding subjects with a history of chronic prostatitis, the relationship between STIs and subsequent PC still remained (adjusted HR 1·99, 95% CI 1·17–3·38).
Table 3. Incidence rate and hazard ratios for prostate cancer in the sample patients without a history of chronic prostatitis during the 5-year follow-up period (n = 11 438)

CI, Confidence interval.
† Hazard ratio was calculated by the stratified Cox regression model (stratified on age group).
‡ Adjustments are made for patients' geographical location, urbanization level, monthly income, history of vasectomy, hypertension, diabetes, hyperlipidaemia, obesity, tobacco use disorder, and alcohol abuse/alcohol dependence syndrome.
** P < 0·01, * P < 0·05.
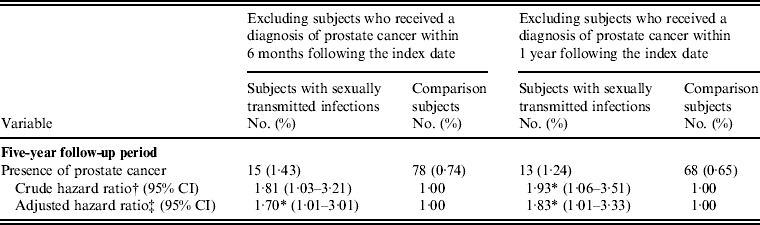
In order to reduce the potential bias caused by the long PC diagnostic latency period, we further performed a sensitivity analysis. Table 4 shows that after excluding subjects who were diagnosed with PC within 6 months and 1 year following the index date, the adjusted HR was 1·70 (95% CI 1·01–3·01) and 1·83 (95% CI 1·01–3·33), respectively. STI was consistently and significantly associated with PC.
Table 4. Sensitivity analysis

CI, Confidence interval.
† Hazard ratio was calculated by the stratified Cox regression model (stratified on age group and the year of index date).
‡ Adjustments are made for patients' geographical location, urbanization level, monthly income, history of vasectomy, hypertension, diabetes, hyperlipidaemia, obesity, tobacco use disorder, and alcohol abuse/alcohol dependence syndrome.
* P < 0·05.
DISCUSSION
Although several studies have explored the link between STI and PC, the results remain inconclusive. To the best of our understanding, this is the first population-based cohort study to investigate the relationship between STI and subsequent risk for PC in an Asian population. In this study, we found that men with a history of STI were more likely to have been subsequently diagnosed with PC than those without, after adjusting for monthly income, geographical location, hypertension, diabetes, hyperlipidaemia, obesity, chronic prostatitis, tobacco use disorder, history of vasectomy, and alcohol abuse/alcohol dependency syndrome. Our results provide further evidence to support the previously reported association between STI and PC by using a population-based study in Taiwan.
Increasing evidence indicates that chronic inflammatory states contribute to carcinogenesis [Reference De Marzo2]. Colorectal carcinoma is associated with inflammatory bowel diseases, oesophageal cancer is associated with reflux esophagitis, hepatocellular carcinoma is associated with viral hepatitis, bladder squamous cell carcinoma is associated with schistosomiasis, and gastric cancer is associated with chronic Helicobacter pylori infection. It is suggested that the longer the inflammation persists, the higher the risk of cancer. Inflammatory cells including neutrophils, macrophages, dendritic cells, mast cells, and lymphocytes are recruited after damage or an infection, and they can secrete a large number of cytokines and chemokines that promote the growth of neoplastic cells and contribute to the onset and progression of cancer [Reference Federico21]. In addition, inflammatory cytokines also regulate the progression and metastasis of tumours. Recently, numerous reports have revealed a potential link between chronic prostatic inflammation and prostate cancer [Reference Sciarra22].
Although the pathogenesis of PC is still not fully understood and several mechanisms have been identified as being involved in its development and progression, there is increasing evidence pointing to the contribution of chronic prostatic inflammation in the development and progression of PC. Chronic prostatic inflammation can be initiated from stimuli arising from bacterial or non-bacterial prostatitis. It is well known that inflammatory infiltrates composed of leukocytes are responsible for the secretion of cytokines. In previous studies, chronic inflammatory conditions and pro-inflammatory cytokine expression have been shown to enhance the activity of IL-6, IL-8, IL-15, and IL-17, which have in turn been suggested to be involved in the development of both benign prostatic hyperlasia and PC [Reference Djavan23, Reference Murphy24]. Taken together, chronic prostatic inflammation could be one of the possible contributing mechanisms associated with PC.
While the results of this study were consistent with previous reports and provide more evidence supporting the association between STIs and subsequent PC, this investigation further found the association to be more apparent after excluding men with a history of chronic prostatitis. This may imply that STIs not involving the prostate tissue may still possibly be involved in the development of PC. In the California Men's Health Study, Cheng et al. found that Latinos had a significantly increased risk of PC associated with a history of STDs [Reference Huang13]. Asian Americans with a history of syphilis and Chlamydia bore a statistically stronger association with PC than those without. These results suggest that racial/ethnic differences exist in terms of the association between STIs and PC risk. Our data support their findings as our study population was an Asian cohort. Sutcliffe et al. suggested that repeated episodes of gonorrhoea may be associated with PC risk [Reference Dennis, Lynch and Torner4]. Gonorrhoea, which is caused by Neisseria gonorrhoeae bacterium, have been shown to induce a chronic inflammatory environment within the prostate through an insufficient immune response, repeated infections, the local production of inflammatory cytokines, reactive oxygen species and recruitment of neutrophils and macrophages that carry inflammatory agents that may induce cell and genome damage leading to increased cellular proliferation.
The strength of our study lies in its longitudinal database and large population size. In addition, over 98% of Taiwan's residents are of Han Chinese ethnicity. While the homogenous population may exempt our study from potential confounding by race, it also means the results may not be generalizeable to other ethnic groups. Nevertheless, this study suffers from a few limitations that should be addressed. The first limitation is the use of ICD coding to diagnose STIs. The details of individual patients including the severity, duration, and medication use were not available. Moreover, we did not have the seropositive status of variable microbiology including Trichomonas vaginalis and Chlamydia trachomatis. Second, individual information such as smoking status, alcohol use, body mass index, PSA screening frequency, and family history of cancer, all of which may contribute to cancer risk and diagnosis establishment, was not available through the administrative dataset. However, this present study took tobacco use disorder, obesity, and alcohol abuse/alcohol dependency into consideration in the regression model.
Third, several lines of evidence have suggested that green tea may prevent PC. In particular, green tea is very popular in Asian populations. However, this dataset lacks information on green tea consumption. The findings of this study could be compromised by the difference in green tea consumption between men with and without a history of STI.
Finally, this investigation may suffer from a surveillance bias in which patients with one condition (STI) were more likely to be diagnosed with a separate and possibly unrelated condition (PC) purely based on their increased exposure to the medical community. Therefore, it is possible that more PC was detected in patients with STI due to increased imaging, which may have contributed to the association detected in this study. However, the unique combination of characteristics of Taiwan's NHI helps guard against the possibility that there was a significant difference in healthcare-seeking behaviour between cases and controls. The Taiwan NHI programme boasts universal coverage, comprehensive benefits, very low out-of-pocket payments (around US$5), and access to any of a wide variety of medical institutions well distributed throughout the country. In the case of PC specifically, the NHI offers free screening examinations for prostate-specific antigen (PSA) every 3 years for men aged >45 years. Therefore, on account of both the low cost and convenience of accessing medical care under the NHI system, as well as the NHI-sponsored PSA screening programme, most older Taiwanese males will have regular check-ups, not hesitate to seek medical attention in the event of experiencing any symptoms or discomfort, and regularly undergo PSA screening. However, despite these factors it remains very possible that our estimation of association can be partly explained through surveillance bias.
Despite these limitations, this study was able to contribute risk estimates to the measures of association reported in the literature. Our results call attention to the potential health impact of STIs for patients. Future experimental and epidemiological examination in other regions or races is needed to shed light on possible and more precise explanatory mechanisms linking STIs and PC.
ACKNOWLEDGEMENTS
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan and managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
DECLARATION OF INTEREST
None.