INTRODUCTION
A novel avian influenza A(H7N9) virus that infects humans was identified in China on 30 March 2013 [Reference Gao1]. According to China National Health and Family Planning Commission reports, 136 confirmed cases of human infection with influenza A(H7N9) virus, including 45 deaths (33%), occurred in mainland China as of 31 October 2013. Human infections with avian influenza A subtypes H7N2, H7N3, and H7N7 have previously been reported worldwide [Reference Belser2]. However, these 2013 cases of influenza A(H7N9) infection represent the first time that a low pathogenic avian influenza virus has been associated with human fatalities [Reference Uyeki and Cox3]. Estimation of the incubation period of influenza A(H7N9) infection is important for understanding the mechanisms of transmission and for control measure recommendations [Reference Lessler4, Reference Reich5].
At the beginning of the influenza A(H7N9) outbreak, the incubation period was generally quoted as being between 1 and 7 days. This estimate was based on influenza A(H5N1) infection. Results of a preliminary study then indicated that the incubation period for influenza A(H7N9) virus was 1–10 days [Reference Li6]. However, there is limited availability of detailed data on the incubation period of confirmed human influenza A(H7N9) cases. We conducted an epidemiological study in Jiangsu Province, one of the first locations where the novel influenza A(H7N9) virus emerged in China. The objective of the study was to collect detailed exposure history data and to provide a more accurate estimate of the incubation period for human influenza A(H7N9) cases.
METHODS
Case definitions
All confirmed human influenza A(H7N9) cases that occurred in Jiangsu Province were included in this study. Case definition was in accord with guidelines issued by the National Health and Family Planning Commission of the People's Republic of China [7]. As of 30 September 2013, 28 confirmed cases of human infection with influenza A(H7N9) virus were reported in Jiangsu. Nine (32%) of these cases were fatal.
Nasopharyngeal/oropharyngeal swabs or tracheal aspirates were collected from each patient. Specimens were sent to the laboratory in viral transport medium as soon as possible. Real-time reverse transcriptase–polymerase chain reaction (rRT–PCR) and inoculation into Madin–Darby canine kidney cell (MDCK) culture for virus isolation were used to assay the samples. Results of the rRT–PCR assay revealed that all of the 28 patients were positive for infection with influenza A virus subtype H7N9. Virus isolation assays revealed that 17 (61%) patients were infected with the virus.
Data collection
We performed a retrospective epidemiological investigation from 25 April to 17 May 2013 that included information on demographic characteristics and poultry-related exposure histories for the 2-week period before the onset of symptoms. The data included the date, frequency, and mode of exposures to poultry or birds. Poultry-related exposure was classified into two types: direct contact with poultry or birds, and poultry-related environmental exposure. Direct contact with poultry or birds included direct contact in the wet market (i.e. culling and slaughtering), direct contact in the home (i.e. cleaning, processing, cutting), and occupational exposure. Poultry-related environmental exposure was defined as visiting a live poultry market, visiting a bird market, buying freshly killed poultry or birds at a live poultry market, or exposure to chickens or pigeons reared in the neighbourhood. The presence of underlying medical conditions (i.e. chronic diseases, including hypertension, diabetes, cancer, chronic bronchitis, rheumatic arthritis, and coronary artery disease) was recorded.
A family member (usually the spouse) was used as a proxy for cases that died from the infection or were too ill to respond to the investigation. Fourteen (50%) patients provided their own exposure history. Exposure history was obtained from the proxies of the remaining 14 (50%) cases (i.e. the nine fatal cases and the five severe cases). The investigation was conducted by trained students from the Chinese Field Epidemiology Training Programme and by staff from Jiangsu Provincial Centre for Disease Control and Prevention.
The study was approved by the Ethics Committee of Jiangsu Provincial Centre for Disease Control and Prevention. Informed consent was obtained from all of participants before the investigation began.
Statistical analysis
Data were analysed using Stata/SE v. 9.0 for Windows (StataCorp LP, USA). We used descriptive statistics to summarize the demographic characteristics and exposure history. The incubation period was defined as the time from exposure to illness onset. To estimate the incubation period we used previously described methods [Reference Huai8]. For the cases with exposures on multiple days, we calculated each case's median incubation period and then calculated the overall median and range of the distribution of the median incubation periods. The minimum and maximum incubation periods were estimated using the last and first known exposure days, respectively. The overall incubation period for all cases was estimated by determining the median of the distribution of the cases’ median incubation periods. Comparisons of overall median incubation period in different subgroups were performed using Wilcoxon's rank-sum test. All statistical tests were two-sided, with a significance level of α = 0·05.
RESULTS
Epidemiological characteristics
All of the 28 confirmed human influenza A(H7N9) cases observed in Jiangsu Province were included in the study. Patients’ age varied from 15 to 85 years (median 55 years). Twelve (43%) patients were elderly (⩾60 years), and 20 patients (71%) were male. Twenty-five (89%) patients lived in an urban area. Three patients were poultry workers, two worked in a live poultry market (slaughtering and selling poultry), and one worked in poultry transportation. Thirteen (46%) of the patients had chronic medical diseases.
Twenty-two (79%) of the patients had a history of exposure that occurred within 2 weeks before illness onset. Ten (45%) of these patients had direct contact with poultry or birds, 11 (50%) were exposed to a poultry-related environment, and one (5%), a 32-year-old female patient, was exposed to an influenza A(H7N9) patient (her father) in her role as caregiver (Table 1). This last patient represented a case of possible human-to-human transmission [Reference Qi9]. Sixteen (73%) of the 22 patients had a history of exposure to poultry or other types of birds, including chickens, pigeons, ducks, quails, and pet birds.
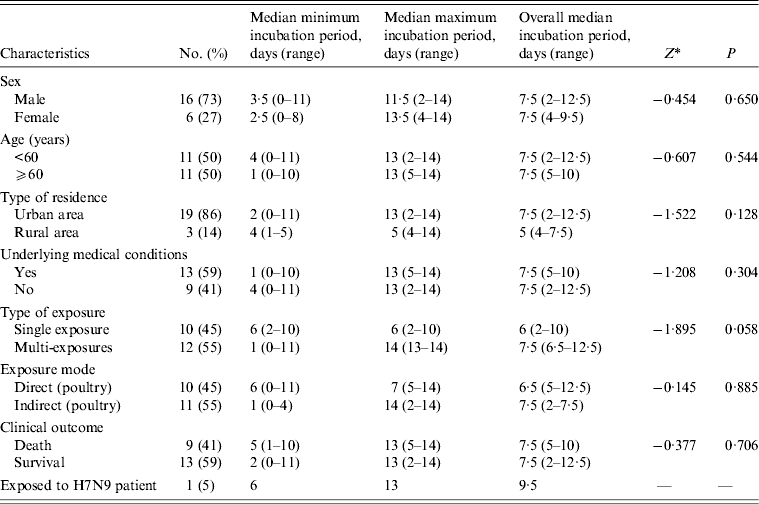
Table 1. Epidemiological characteristics of 28 patients infected with avian influenza A(H7N9) virus in Jiangsu Province, China, 2013

* Two of the three poultry workers worked in a live poultry market (slaughtering and selling poultry) and one worked in poultry transportation.
† One each of the following occupations: student, civil servant, laboratory technician, university professor, self-employed businessman, and security.
‡ Chronic diseases, including hypertension, diabetes, cancer, chronic bronchitis, rheumatic arthritis, and coronary artery disease.
Estimation of incubation periods
The interval in days between exposure and onset was used to estimate the incubation periods for the 22 cases. The six cases with no poultry-related exposure history were excluded from the analysis. The median incubation period was 6 days (range 2–10 days) for the 10 (45%) cases with a single exposure and 7·5 days (range 6·5–12·5 days) for the 12 (55%) cases with multiple exposures. The difference between the two groups was not statistically significant (Z = −1·895, P = 0·058). When data for single and multiple exposure days were combined, the overall median incubation period for patients who had direct contact with poultry or birds was 6·5 days (range 5–12·5 days). This result was not significantly different from the median incubation period for patients who were exposed to a poultry-related environment (7·5 days, range 2–7·5 days) (Z = −0·145, P = 0·885). None of the other differences in overall median incubation period in survivors and deaths, males and females, different age groups, urban and rural cases, and in cases with and without underlying medical conditions, were statistically significant (Table 2). The incubation period for the patient who was exposed to her infected father was 6–13 days. The upper limit of the minimum incubation period was 11 days, because one patient developed symptoms 11 days after his last exposure to poultry.
Table 2. Estimated incubation periods for 22 patients infected with avian influenza A(H7N9) virus in Jiangsu Province, China, 2013
* Comparisons of overall median incubation period in different subgroups were performed using Wilcoxon's rank-sum test.
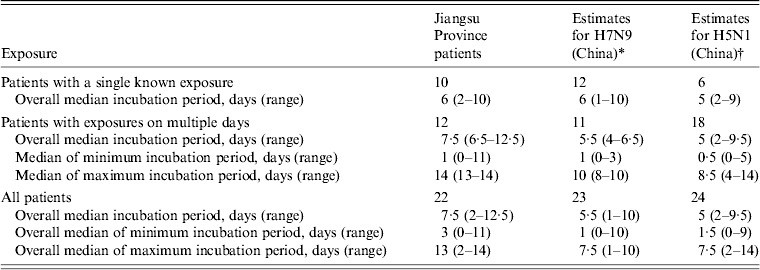
We compared our estimated incubation period to the results from previous studies that estimated incubation periods for influenza A(H7N9) and A(H5N1) infection. The data were cited from studies that used the same estimation methods that we used in this study (Table 3). In our study, the overall median incubation period for H7N9 virus was 7·5 days (range 2–12·5 days). It was 5·5 days (range 1–10 days) in a preliminary study in China [Reference Li6]. The incubation period for H5N1 cases in China was 5 days (range 2–9·5 days) [Reference Huai8].
Table 3. Comparison of estimated incubation periods
* These data are from a preliminary study [Reference Li6].
† These data are from a previously published study [Reference Huai8].
DISCUSSION
There is a substantial amount of uncertainty regarding the length of the incubation period for novel avian influenza A(H7N9) virus infection. Earlier attempts to estimate it were primarily based on available exposure history data, which were collected from national reporting system databases consisting of data from local Centres for Disease Control and Prevention [Reference Li6, Reference Cowling10]. By contrast, we conducted an in-depth, epidemiological investigation for all of the reported cases in the Jiangsu outbreak and obtained a more detailed and accurate estimate of the incubation period.
Genetic evidence indicates that poultry serve as the reservoir for influenza A(H7N9) virus infection [Reference Chen11, Reference Han12]. Results of epidemiological investigations also support the hypothesis that influenza A(H7N9) virus-infected poultry are a source of infection [Reference Bao13, Reference Han14]. Seventy-nine percent of the influenza A(H7N9) patients had direct contact with poultry or a history of exposure to a live poultry market before illness onset. This result is consistent with the result of an earlier study [Reference Li6]. Visiting a live poultry market in China has been identified as a risk factor for influenza A(H5N1) [Reference Mounts15, Reference Zhou16] and for novel influenza A(H7N9) [Reference Ai17] virus infection. The apparent reduction in human cases after the closure of live poultry markets also suggests that exposure to live poultry was the main source of human infection. Therefore, we regarded exposure to live poultry or to poultry-related environments as the relevant exposure that should be used to estimate incubation periods.
Our estimate of 7·5 days for the overall median incubation period for human infection with avian influenza A(H7N9) is much longer than the 3·1 days estimated by Cowling et al. [Reference Cowling10] who used a completely different method (Weibull model) for their estimate. However, the method used in our study was the same as was used to estimate the incubation period for influenza A(H5N1) [Reference Qi9]. Compared to the results of another study conducted in China [Reference Li6], which used the same method to estimate the influenza A(H7N9) incubation period that we used, the median incubation period in patients with a single known exposure was much more consistent. However, the overall median incubation period estimated in our study was 2 days longer (7·5 days vs. 5·5 days). The estimated upper limit for the maximum incubation period of 12·5 days was also longer than the 10-day incubation period estimated in the earlier study. Our result of the influenza A(H7N9) incubation period was also obviously longer than the estimate for seasonal influenza. The results of a systematic review suggested that the median incubation period for influenza A is 1·4 days [Reference Lessler18].
Results of previous studies of influenza A(H5N1) have indicated that the median incubation period is 3 days in Vietnam [Reference Tran19] and Thailand [Reference Areechokchai20], and the mean time between exposure and the onset of symptoms is 5 days in eastern Turkey [Reference Oner21]. Compared to the results from a study on incubation period for influenza A(H5N1) in China [Reference Huai8], upon which our estimation method is based, our estimated overall median incubation period for influenza A(H7N9) was 2·5 days longer [7·5 days (range 2–12·5 days) vs. 5 days (range 2–9·5 days)]. For influenza A(H5N1) clusters in which limited human-to-human virus transmission probably occurred, the incubation period appeared to be 3–9 days [Reference Wang22–Reference Kandun24], but in our study it was 6–13 days.
The incubation period is an important aspect of disease natural history. Knowledge regarding the incubation period is important for the surveillance and control of infectious disease. Our findings suggest that the incubation period for novel influenza A(H7N9) infection may be longer than estimated by previous studies for influenza A(H7N9) and A(H5N1). The maximum incubation period could be longer than the 7 days that has been used as the maximum incubation period thus far [7], and longer than the 10 days estimated in a previous study [Reference Li6]. We also compared the differences in median incubation period in different groups stratified by demographic characteristics and exposure history, but they were not statistically significant. H7N9 virus is a novel reassortant avian-origin influenza A virus. The reason that it may have a longer incubation period than influenza A(H5N1) remains unresolved. More studies on the pathogenetic mechanisms of influenza A(H7N9) are needed.
For reasons of biological plausibility, the maximum time from first exposure to illness onset was limited to 14 days. Exposure history for the 2 weeks before illness onset was collected for each patient. The median incubation period may be overestimated for the patients who reported multiple exposures. Estimates of the incubation period for cases in which a single exposure occurred might be more accurate. Based on our results, however, we highlight the need for public health authorities to extend the period of medical surveillance from 7 days to 10 days. Case definitions should be updated, and the quarantine period for close contacts should be at least 10 days. The CDC's interim guidance on case definitions has ‘within <10 days of illness onset’ as the medical surveillance period [25]. Further studies that include more samples and accurate estimates of the incubation period distribution are needed, because the estimated incubation period for some of the cases in our study was more than 10 days.
Several potential limitations of this study should be noted. First, only a small number of cases were available, which reduced the statistical power of the study. Therefore, the results should be interpreted with caution. Second, for some cases, proxies responded to the survey, which may have introduced some uncertainty about exposure dates. This issue may have been especially relevant when infection occurred in patients with multiple days of exposures. Third, the difference in the incubation period between our study and the influenza A(H5N1) study presented in Table 3 could not be tested for statistical significance because the raw data used for the estimate of influenza A(H5N1) incubation times were not available.
In conclusion, our findings suggest that the incubation period for the novel influenza A(H7N9) may be longer than previously estimated. This possibility highlights the necessity for public health authorities to extend the period of medical surveillance from 7 days to 10 days. Our results contribute to a better understanding of novel influenza A(H7N9) infection and provide important information that will aid in the surveillance and control of this disease.
ACKNOWLEDGEMENTS
We thank the Centres for Disease Control and Prevention of Nanjing, Suzhou, Wuxi, Zhenjiang, Changzhou, Yancheng, Yangzhou, and Suqian cities in Jiangsu Province for coordinating our field investigations and collecting the data. This work was supported by the Jiangsu Province Health Development Project with Science and Education (M.H.Z., H.W., ZX201109; X.Q., RC2011084; Y.F.Z., RC2011085), a grant from the National Ministry of Science and Technology Emergency Research Project on human infection with avian influenza H7N9 virus (F.Y.T., no. KJYJ-2013–01–02) and the Chinese Field Epidemiology Training Programme (Y.H., D.F.R, G.Q.S., T.S.).
DECLARATION OF INTEREST
None.






