INTRODUCTION
Neisseria meningitidis, also known as the meningococcus, is a gram-negative bacterium that typically lives as a commensal species in the human nasopharynx. Humans are the only natural host for meningococci, which are generally non-invasive, colonizing without causing any harm. Rarely, however, meningococci invade the nasopharyngeal epithelia of the host and enter the bloodstream, causing life-threatening illnesses [Reference Hill1]. Meningococcal infection can cause many minor diseases but the most common and life-threatening of these are meningococcal meningitis and meningococcaemia [Reference Pathan, Faust and Levin2]. In spite of its usual commensal state, N. meningitidis is nevertheless recognized as an important cause of bacterial meningitis globally, and one of the top 10 infectious killers worldwide [Reference Stephens3].
Carriage rates of N. meningitidis vary around the world, but typically range from about 10%–25%, with higher rates of carriage seen among adolescents and young adults [Reference Yazdankhah and Caugant4]. The factors that cause benign colonization to result in disease are incompletely understood, but are likely a combination of host, bacterial, and environmental factors. N. meningitidis is highly variable; carriage-associated strains in particular are very diverse, while invasive strains are often associated with a small number of genetically defined hypervirulent lineages [Reference Caugant5, Reference Weber6]. Occasionally, sporadic outbreaks or enduring epidemics, lasting years or decades, can occur. Between 1991 and 2008, New Zealand experienced a prolonged serogroup B epidemic, with the majority (>85%) of disease cases during the epidemic period caused by a single strain type [Reference Devoy, Dyet and Martin7, Reference Dyet8]. In response to the epidemic, beginning in 2005, a strain-specific outer membrane vesicle vaccine, MeNZB™, was introduced to control the epidemic [Reference Oster9].
Meningococci are frequently classified by three surface structures, including their outer capsule composition and PorB and PorA major outer membrane proteins, which designate serogroup, serotype, and serosubtype, respectively. The N. meningitidis capsule is a well-recognized and important virulence factor; it surrounds and provides protection to the bacterial cell [Reference Stephens3]. The capsule polysaccharides vary in chemical structure, and provide a basis for serogroup classification of N. meningitidis. At least 13 different capsule serogroups have been described, with five serogroups, including A, B, C, W135, and Y, most frequently causing disease [Reference Yazdankhah and Caugant4]. The meningococcal capsule plays an important role in protecting the bacterium from phagocytosis when it is in the bloodstream [Reference Read10]. Indeed, the capsule is necessary for survival of meningococci in the blood [Reference Kahler11], and may also enhance transmission between hosts by increasing the organism's resistance to desiccation [Reference Virji12].
Transmission of meningococci remains relatively poorly understood. It is generally considered that meningococci do not survive outside the host, and that transmission, via respiratory droplets (e.g., coughing and sneezing) or intimate kissing, requires relatively close contact between hosts [Reference Stephens3, Reference Yazdankhah and Caugant4, Reference Musher13, Reference MacLennan14]. Although no firm evidence exists that indirect transfer of meningococci can occur via surfaces (fomites), there is some evidence that sharing drinking vessels, food, or a pacifier, may be a risk factor for transmission [Reference Baker15].
Despite the prevailing view that transmission requires close contact between hosts, survival of meningococci on fomites was demonstrated over seven decades ago by Miller and Schad who reported survival of virulent meningococci up to 10 days after drying on glass and up to 7–8 days after drying on wood or cotton cloth [Reference Miller and Schad16]. More recently, two of us reported survival on glass and plastic of seven isolates from B, C and W135 serogroups, including the New Zealand, Cuban and Norwegian epidemic B strains [Reference Swain and Martin17]. Survival on glass was up to 6–5 days. Subsequently, Tzeng et al. reported survival for up to 3 days of N. meningitidis A, B and C strains dried on glass, plastic or metal and concluded that encapsulation did not confer greater survival [Reference Tzeng, Martin and Stephens18].
There is need for more extensive knowledge of whether some strains have greater ability to survive on surfaces, as this has potential implications for transmission [Reference Swain and Martin17]. We therefore characterized the survival of isolates from B, C and W135 serogroups, including New Zealand B:4:P1.7-2,4 epidemic strain isolates, after drying on glass coverslips. We also compared survival of B:4:P1.7-2,4 carriage and disease-associated isolates, and compared the survival of serogroup C and W135 isolates that are related but altered by a capsule switch [Reference Beddek19].
METHODS
Bacterial strains and growth conditions
New Zealand isolates were from B, C and W135 serogroups and were associated with the carriage state or invasive disease (Table 1). Strains were routinely typed at the Institute of Environmental Science and Research (ESR) using serological and sequencing methods to determine serogroup, serotype (porB allele) and serosubtype (porA allele) [Reference Abdillahi and Poolman20]. All of the disease-associated strains were isolated from blood or cerebrospinal fluid of patients with clinical cases of disease and were part of the Meningococcal Reference Collection, collected and maintained by ESR as part of the surveillance of meningococcal disease in New Zealand. The carriage-associated isolates (NZA prefix) were collected as part of a throat swab survey carried out on New Zealand Army recruits. The disease-associated strains included the New Zealand B:4:P1.7-2,4 epidemic isolate, NZ98/254, from which the MeNZB™ vaccine had been developed [Reference Oster9]. N. meningitidis isolates representative of the Norwegian and Cuban serogroup B epidemics (isolates H44/76 and Cu00162 respectively) [Reference Brooks21, Reference Climent22] were also examined. All isolates of the strain type B:4:P1.7-2,4 belonged to the ST-41/44 clonal complex, though sequence types were not known for the remaining isolates.
Table 1. New Zealand N. meningitidis disease-associated and carriage strain types and isolates

New Zealand isolates were either disease-associated, cultured from blood or cerebrospinal fluid of patients with clinical cases of disease (strains with an NZ prefix) from the New Zealand national surveillance program at the Institute of Environmental Science and Research Ltd, or carriage isolates (NZA prefix), recovered from a throat swab survey of New Zealand Army recruits. Strain types are presented according to convention serogroup: serotype (PorB allele): serosubtype (PorA allele).
nt, nontypable.
Bacterial culturing was carried out as previously described [Reference Swain and Martin17]. Briefly, bacteria were grown on 5% Columbia sheep blood agar (CBA) plates (Fort Richard Laboratories, Auckland, New Zealand). Starter cultures of each strain were prepared in Brain-Heart Infusion (BHI) broth and stored in the presence of 15% glycerol at −80 °C in 1 ml aliquots until used. Growth curves of the starter cultures were tested to confirm the cultures were not adversely affected by freezing.
Inoculation of fomites
Thawed starter cultures were used to inoculate 25 cm2 tissue culture flasks containing 5 ml BHI, and were incubated in a Heraeus HERAcell incubator with 5% CO2 at 36 °C and 95% RH for 4 h. The suspensions were then diluted with BHI to OD 0·1 at 650 nm, and then to a final concentration of 3 × 104 colony forming units (CFU)/ml in Defined Medium Mucin (DMM), an artificial saliva kindly donated by Chris Sissons and Lisa Wong [Reference Wong and Sissons23]. Twenty-five μl (approximately 750 CFU) aliquots of the DMM bacterial suspensions were inoculated onto UV-sterilised 18 mm × 18 mm glass cover slips and left to dry in open sterile plastic Petri dishes (10 coverslips per dish). The zero time point was defined as the time at which all cover slips in the first Petri dish were dry. After drying, batches of cover slips were incubated either at ambient temperature and humidity, or under controlled environmental conditions. Controlled conditions included 30% or 22% relative humidity at 30 °C in an environmental chamber (Contherm Scientific, Lower Hutt, New Zealand; model 4150). Relative humidity (RH), defined as the amount of water vapour as a percentage of that required for saturation at a given temperature, was measured using a portable temperature and humidity meter.
Recovery of meningococci from fomites
Recovery of meningococci from fomites was measured as previously described [Reference Swain and Martin17]. Bacteria were recovered by pressing duplicate cover slips onto the surface of pre-warmed CBA plates for 2 min. The CBA plates were inspected for bacterial growth after 24 h and 48 h of incubation (36 °C, 5% CO2, 95% RH) and the colonies were manually counted. As a control to ascertain the number of CFU inoculated onto each cover slip, the 3 × 104 CFU/ml DMM suspensions were diluted fivefold and 25 µl aliquots were plated onto each of three CBA plates. The plates were incubated overnight, and the colonies were counted and the average inoculations calculated the following day. For each experiment, surviving meningococci were measured at zero time immediately after drying onto glass coverslips, then after 1, 2, 3, 4, 19, 22, 25, 28 h incubation, and at several times each day thereafter, until all of the time points measured in 1 day failed to yield any viable meningococci. Recovery was used to define survival, although it is possible that additional viable bacteria may not have been transferred from the coverslips. The press method was used, however, to represent potential environmental transmission through contact with meningococci on fomites (see Discussion).
Statistical analysis
All data analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL). Statistical modelling of the survival of meningococci was carried out using binary logistic regression [Reference Nick and Campbell24]. The survival results at each time point were used to model the dependence of the probability of survival on time using the following equation:
where x is time, p is the probability of survival for an individual bacterium,
![]() $\hat \alpha $
is the estimate of the y-intercept, and
$\hat \alpha $
is the estimate of the y-intercept, and
![]() $\hat \beta $
is the estimate of the coefficient of time and describes the gradient of the curve, ln(p/1 − p) is the log of the odds ratio that the bacterium is alive. Each data point in each survival experiment was fitted to a separate logistic regression model.
$\hat \beta $
is the estimate of the coefficient of time and describes the gradient of the curve, ln(p/1 − p) is the log of the odds ratio that the bacterium is alive. Each data point in each survival experiment was fitted to a separate logistic regression model.
For each experiment an estimate of β
![]() $(\hat \beta )$
was obtained that describes the relationship between survival and time for that condition and strain. The
$(\hat \beta )$
was obtained that describes the relationship between survival and time for that condition and strain. The
![]() $\hat \beta $
s can be likened to a death-rate. A negative number indicates a decreasing gradient, and the lesser the number the steeper the curve and the greater the death-rate. Non-parametric Mann–Whitney U two-sample rank-sum analysis of the median
$\hat \beta $
s can be likened to a death-rate. A negative number indicates a decreasing gradient, and the lesser the number the steeper the curve and the greater the death-rate. Non-parametric Mann–Whitney U two-sample rank-sum analysis of the median
![]() $\hat \beta $
s was used for pairwise comparisons of survival.
$\hat \beta $
s was used for pairwise comparisons of survival.
RESULTS
Survival under ambient conditions
Initially, survival on glass of the New Zealand epidemic isolate NZ98/254 was examined under ambient conditions in the laboratory. As previously reported, there was an initial rapid decay in survival followed by more gradual loss (Fig. 1) until a maximum survival time, after which viable organisms were not recovered [Reference Swain and Martin17]. Concurrently with these experiments, survival of NZ98/254 was briefly compared with three other strains under ambient conditions (Table S1). Each strain survived desiccation but there were differences in survival, including initial survival at time zero and maximum survival times. Mean initial recovery rates at time point zero, immediately after desiccation onto glass coverslips, varied between 7% and 24%. For the two B:4:P1.7-2,4 strains, NZ98/254 had a maximum survival time of 100 h (n = 22), while the NZ03/280 isolate had a maximum survival 91 h (n = 7). The Cuban strain Cu00162 had a maximum survival 94 h (n = 2). In contrast, the W:nt:P1.18-1,3 isolate NZ07/024 had lower maximum survival of 52 h (n = 2). Inspection of the median and mean
![]() $\hat \beta $
values (Table S2) that describe the relationship between survival and time, indicated that the greater values of
$\hat \beta $
values (Table S2) that describe the relationship between survival and time, indicated that the greater values of
![]() $\hat \beta $
were associated with longer maximum survival. For example, the mean
$\hat \beta $
were associated with longer maximum survival. For example, the mean
![]() $\hat \beta $
values for NZ98/254 and NZ07/024 were −0·29 and −0·62 respectively, corresponding to the greater survival of NZ98/254.
$\hat \beta $
values for NZ98/254 and NZ07/024 were −0·29 and −0·62 respectively, corresponding to the greater survival of NZ98/254.
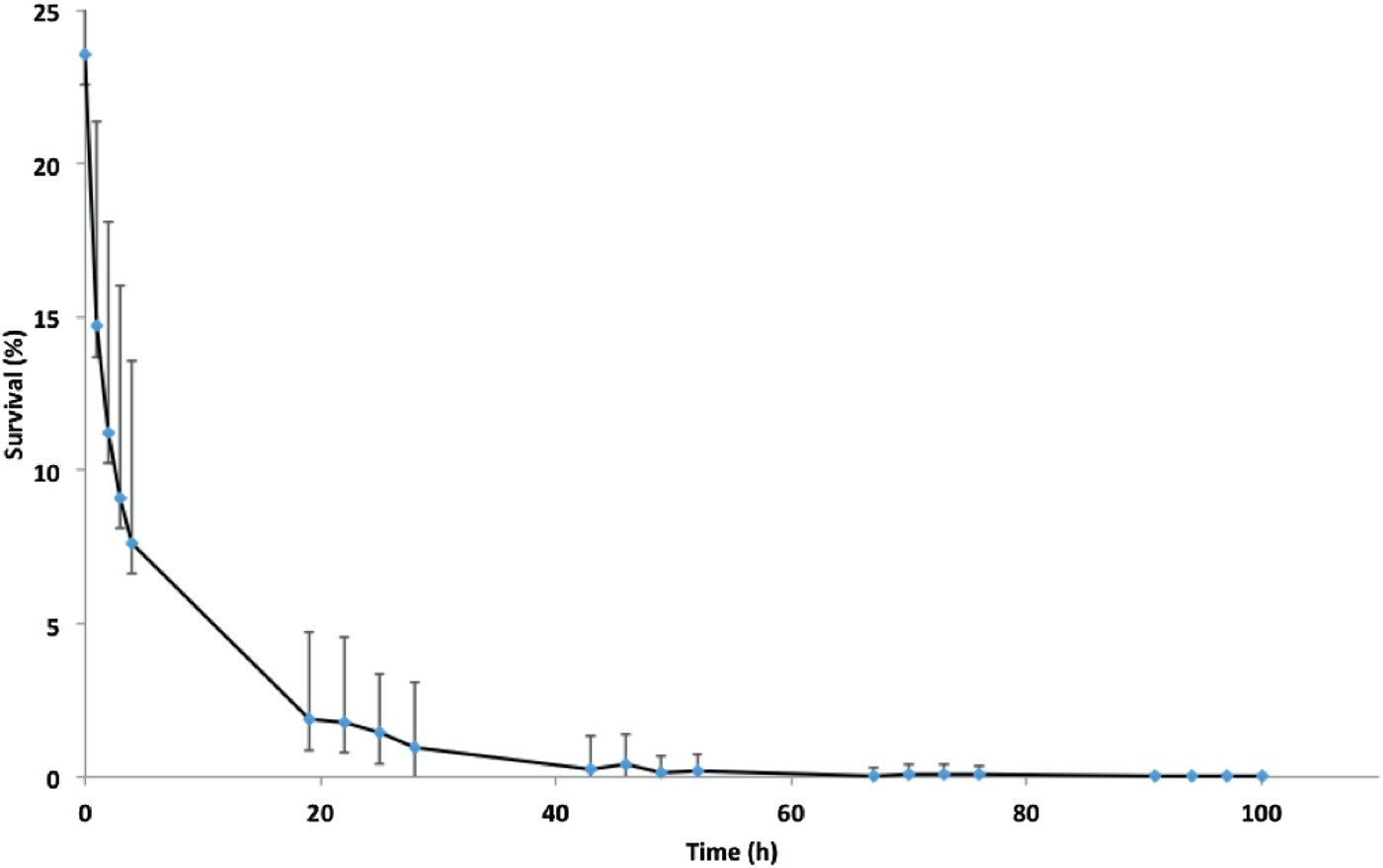
Fig. 1. Survival of isolate NZ98/254 on glass under ambient conditions of temperature and humidity in the laboratory. Data points are means of 22 experiments carried out over a 12-month period. Mean survival at zero time immediately after drying was 23·6 ± 8·6% of initial inoculations, followed by approximately 70% further loss at 4 h, 94% loss at 24 h and further loss until viable organisms were not recovered at 100 h. Error bars represent standard deviations, which are large due to seasonal variation in survival (Fig. 2).
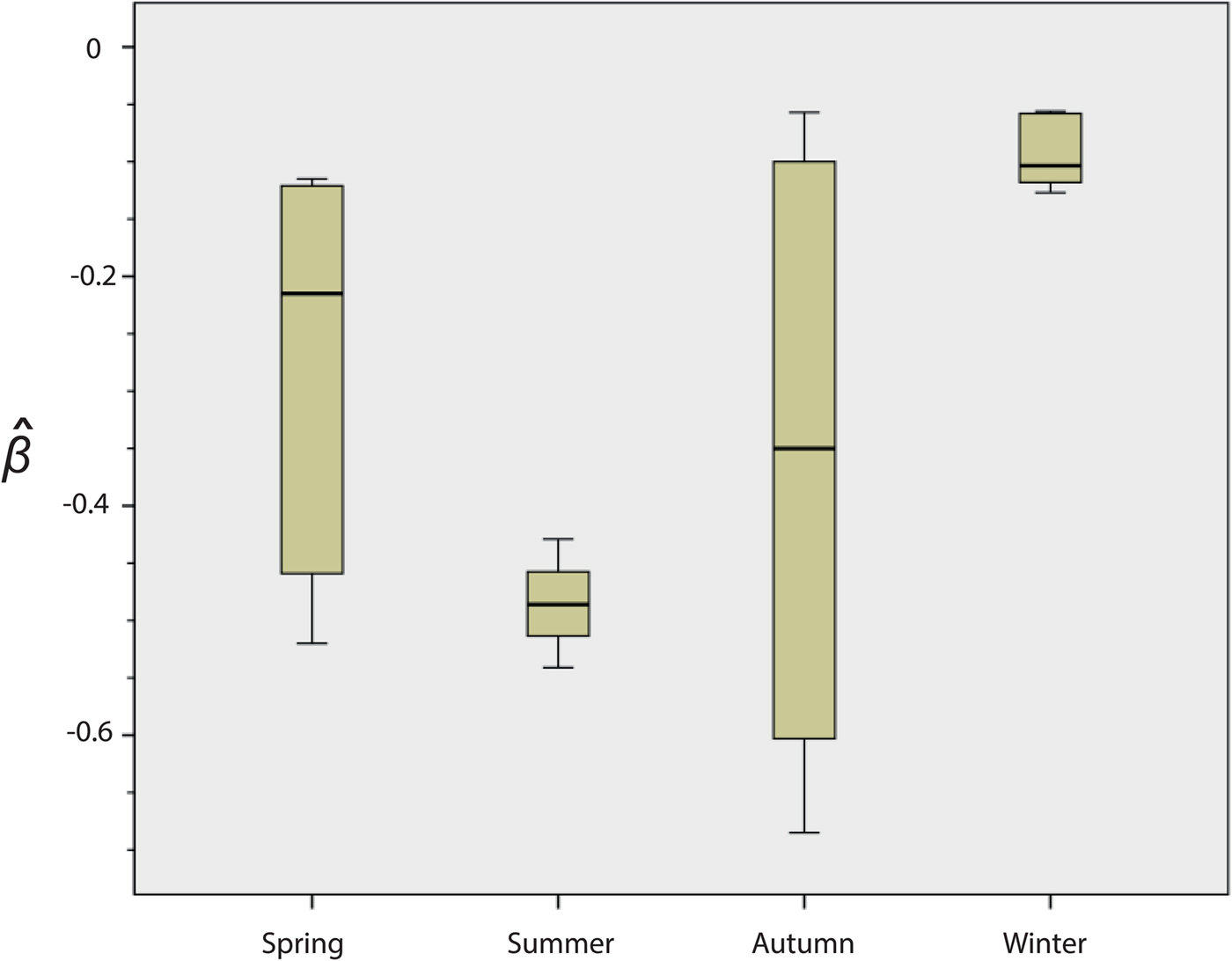
Fig. 2. Seasonal variation in the survival of strain NZ98/254. Results are means for the 22 experiments: spring n = 5, summer n = 4, autumn n = 7, winter n = 6. The y-axis indicates the death rate (
![]() $\hat \beta ).\; $
There were significant differences in survival between winter and summer (P = 0·010) and winter and spring (P = 0·031). In both cases, survival was higher in the winter. Differences were not significant between spring and autumn (P = 1·000) or between spring and summer (P = 0·190).
$\hat \beta ).\; $
There were significant differences in survival between winter and summer (P = 0·010) and winter and spring (P = 0·031). In both cases, survival was higher in the winter. Differences were not significant between spring and autumn (P = 1·000) or between spring and summer (P = 0·190).
A review of the results of these initial experiments suggested that there were seasonal differences in the ability of NZ98/254 to survive desiccation (Fig. 2 and Fig. S1). Mann–Whitney analysis of the
![]() $\hat \beta $
values found significant differences in survival of NZ98/254 between winter and summer (P = 0·010), and winter and spring (P = 0·031). In both cases, survival was higher in the winter. The differences were not significant between spring and autumn (P = 1·000) or between spring and summer (P = 0·190), likely due to the high variability in survival in spring and autumn. Survival of NZ98/254 was greatest in winter and least in summer, which parallels the increased incidence of meningococcal disease in winter and spring [Reference Martin, Lopez and Sherwood25]. It was speculated that these differences in survival may have been related to fluctuations in temperature and humidity, which during the course of the year ranged from 16 to 28 °C and 28%–64% RH in the laboratory, with lower temperatures and humidity in winter.
$\hat \beta $
values found significant differences in survival of NZ98/254 between winter and summer (P = 0·010), and winter and spring (P = 0·031). In both cases, survival was higher in the winter. The differences were not significant between spring and autumn (P = 1·000) or between spring and summer (P = 0·190), likely due to the high variability in survival in spring and autumn. Survival of NZ98/254 was greatest in winter and least in summer, which parallels the increased incidence of meningococcal disease in winter and spring [Reference Martin, Lopez and Sherwood25]. It was speculated that these differences in survival may have been related to fluctuations in temperature and humidity, which during the course of the year ranged from 16 to 28 °C and 28%–64% RH in the laboratory, with lower temperatures and humidity in winter.
A preliminary investigation of survival under more controlled conditions was therefore done, with the dried isolates on cover slips incubated in a warm room at 36 °C and 20% RH. Survival was apparently greater than for incubation under ambient conditions, with maximum survival exceeding 200 h for NZ98/254 (n = 8) and Cu00162 (n = 2), and extending to 196 h for NZ03/280 (n = 5) (Table S3). In contrast, dried isolates on glass coverslips placed in a Heraeus HERAcell incubator at 36 °C, 5% CO2, 95% RH were not recovered past 30 min for the two New Zealand strains. Similarly, the Cuban strain Cu00162 showed only 1%–2% survival in the incubator at 30 min, and could not be recovered at 60 min (Table S4).
Because these preliminary data indicated a significant effect of temperature and humidity on recovery of meningococci from fomites, we decided to compare the survival of other isolates under controlled conditions. All subsequent experiments were carried out using dried isolates on coverslips incubated in a temperature and humidity controlled cabinet at 30 °C at either 30% or 22% RH prior to measurement of survival. These low-RH conditions were chosen based on the greater survival of meningococci in the warm room (20% RH, vs. a humidified incubator (95% RH).
Meningococcal survival at 30 °C and 30% RH
The survival of multiple isolates belonging to diverse strain types, plus single isolates of the Cuban and Norwegian epidemic strains, was compared at constant 30 °C and 30% RH. The goal of these experiments was to test whether there were differences in survival that might be related to survival on fomites outside the host.
In general, maximum survival times were greatest for isolates belonging to serogroups B and C, with lowest survival seen for isolates belonging to serogroup W135, although there was variation within each serogroup. Examination of the
![]() $\hat \beta $
values (Table 2) indicated that greater survival was again matched by greater values of
$\hat \beta $
values (Table 2) indicated that greater survival was again matched by greater values of
![]() $\hat \beta $
. In general, survival was greater and less variable compared with ambient conditions. For example, mean
$\hat \beta $
. In general, survival was greater and less variable compared with ambient conditions. For example, mean
![]() $\hat \beta $
values for NZ98/254 were −0·132 ± 0·080 at 30 °C, 30% RH, compared with −0·293 ± 0·221 for ambient conditions.
$\hat \beta $
values for NZ98/254 were −0·132 ± 0·080 at 30 °C, 30% RH, compared with −0·293 ± 0·221 for ambient conditions.
Table 2. Survival of N. meningitidis strains at 30 °C and 30% RH

* Carriage strains. All other isolates were from patients with invasive disease.
Survival statistics for all strains are shown in Table S5. Carriage isolates appeared to survive better than invasive isolates from the B:4:P1.7-2,4 strain type both for maximum survival times and
![]() $\hat \beta $
values (Table 2), with a statistically significant difference in
$\hat \beta $
values (Table 2), with a statistically significant difference in
![]() $\hat \beta $
(Mann–Whitney P = 0·002) between carriage and invasive strains. There was not, however, a significant difference (P = 0·81) between the W:nt:P1.18-1,3 invasive and carriage isolates, probably in part due to the smaller number of strains tested (n = 2 for carriage isolates).
$\hat \beta $
(Mann–Whitney P = 0·002) between carriage and invasive strains. There was not, however, a significant difference (P = 0·81) between the W:nt:P1.18-1,3 invasive and carriage isolates, probably in part due to the smaller number of strains tested (n = 2 for carriage isolates).
Mann–Whitney pairwise analysis of the median
![]() $\hat \beta $
values showed significant differences between W:nt:P1.18-1,3 and the other strains (Table 3). We were, however, particularly interested in comparison of the C:2a:P1.7-2,4 and W:2a:P1.7-2,4 isolates that differed by a capsule switch [Reference Beddek19]. Although the difference in
$\hat \beta $
values showed significant differences between W:nt:P1.18-1,3 and the other strains (Table 3). We were, however, particularly interested in comparison of the C:2a:P1.7-2,4 and W:2a:P1.7-2,4 isolates that differed by a capsule switch [Reference Beddek19]. Although the difference in
![]() $\hat \beta $
values was not significant (P = 0·077), the C:2a:P1.7-2,4 isolates survived better at all time periods (Fig. S2 and Table S6), suggesting that differences in the capsule may have some influence on survival.
$\hat \beta $
values was not significant (P = 0·077), the C:2a:P1.7-2,4 isolates survived better at all time periods (Fig. S2 and Table S6), suggesting that differences in the capsule may have some influence on survival.
Table 3. Mann-Whitney U pairwise analysis of differences in median
![]() $\hat \beta $
between the New Zealand disease-associated strains at 30 °C and 30% RH
$\hat \beta $
between the New Zealand disease-associated strains at 30 °C and 30% RH

Values are: U, Mann–Whitney statistic; P, probability.
Meningococcal survival at 30 °C and 22% RH
Further experiments were carried out with dried isolates on coverslips maintained at 30 °C and 22% RH to examine the effect of altered humidity on survival. A subset of the isolates that had been tested at 30% RH was examined, including two disease-associated and one carriage isolate from the best B:4:P1.7-2,4 and poorest W:nt:P1.18-1,3, surviving strains, plus the Cuban and Norwegian epidemic isolates. Five of the eight strains survived >20% longer under these conditions compared with 30% RH. The Cuban and Norwegian epidemic isolates and NZ98/254 had the greatest survival (Table 4, Table S7), and greater survival at 22% RH (1·6-2·1-fold) than at 30% RH. Although the W:nt:P1.18-1,3 strain NZ04/140 also survived substantially better at 22% than at 30% RH, the other two representatives of serogroup W135 were the poorest survivors and varied little between 22% and 30% RH. The greater survival of B:4:P1.7-2,4 compared with W:nt:P1.18-1,3 isolates at 22% RH was significantly different (P < 0·001), based on comparison of the combined invasive and carriage isolates for each strain type. There were also significant differences in the β values between survival of meningococci at 30% compared with 22% RH (Table S8) for the B:4:P1.7-2,4 and Cuban and Norwegian isolates.
Table 4. Survival of N. meningitidis strains at 30 °C and 22% RH

All isolates were examined in triplicate.
DISCUSSION
This work extends knowledge about survival of N. meningitidis strains dried on glass, and includes differences in survival between strain types, and effects of temperature and humidity on survival. Although there was substantial loss of viable organisms immediately after drying, surviving bacteria could be recovered at least 2 days after drying and some strains were recovered after as many as 8 days, depending on environmental conditions. The press method that we used was chosen to optimize the biosafety associated with working with clinical meningococcal isolates (e.g., avoiding aerosolization of the isolates), but reflects the fact that transmission requires both viable bacteria and detachment from surfaces. Similarly, several types of bacteria have been described to enter a ‘viable but nonculturable’ state when exposed to environmental stresses. Although this state has not been described for meningococci, we cannot rule out the possibility that viable but non-culturable bacteria remained on the cover slips following dessication.
Effects of temperature and humidity on survival
Initial experiments showed that NZ98/254 and two other disease-associated serogroup B isolates survived up to 3–4 days in the dried state, under varying conditions of ambient temperature and humidity in the laboratory. In comparison, the serogroup W135 NZ07/024 isolate was more susceptible to death after drying. A follow-up survey of the effects of environmental conditions showed that survival of the B isolates was greater, compared with ambient conditions, when the dried bacteria were kept in a warm room at approximately 36 °C, 20% RH, but was less than 1 h after incubation at 36 °C, 95% RH. With the exception of two of the poorer surviving W:nt:P1.18-1,3 isolates, the survival of all the strains we tested was greater at 22% RH compared with 30% RH.
Temperature and humidity are known to affect the survival of some other bacteria dried on fomites [Reference McEldowney and Fletcher26–Reference Lopez29]. For example, Jawad et al. found differences among microbial strains for survival after drying, and distinguished between sensitive, moderately resistant and resistant strains that survived 0–5, 6–10, and ⩾11 days, respectively [Reference Jawad27]. They reported that moderately resistant Acinetobacter survived better at 31% or 90% RH compared with 10% RH, and speculated that nosocomial infections of Acinetobacter might be transmitted in clinical settings after drying on surfaces. In contrast, methicillin-resistant Staphylococcus aureus survived better at 16% RH compared with 45–55% RH [Reference Coughenour, Stevens and Stetzenbach28].
The seasonal variation in survival, observed for NZ98/254, was not systematically investigated for other strains at a range of ambient conditions, but the differences in survival between 30% and 22% RH at 30 °C confirmed that humidity affected the survival of some dried meningococci, although this effect may vary at different temperatures. Because the recovery of NZ98/254 was found to have a strong seasonal variation, we opted to control for temperature and humidity prior to assessing the recovery of additional isolates from fomites.
Strain differences in survival
N. meningitidis serogroups differ in the structure of their extracellular polysaccharide capsules, which, in the case of the serogroups B and C, are homopolymers of N-acetyl-neuraminic acids (sialic acids), while the W135 capsule contains heteropolymers of galactose and N-acetyl-neuraminic acid [Reference Hill1]. The capsules of invasive strains have protective roles within the host, including avoidance of phagocytosis and complement activation and have been implicated in colonization and invasion [Reference Stephens3, Reference Read10]. We were therefore interested in whether capsule differences might also be implicated in differences in survival and potential for transmission, outside the host.
Comparisons of survival at constant temperature and humidity showed differences among serogroups and between carriage and disease-associated strains. In general the B:4:P1.7-2,4 and C:2a:P1.7-2,4 strains survived better than the W:nt:P1.18-1,3 and W:2a:P1.7-2,4 isolates at 30 °C and 30% RH. Additionally, for the New Zealand B:4:P1.7-2,4 strain type, carriage isolates from nasopharyngeal swabs survived better than the invasive isolates from blood or CSF. This difference in survival between carriage and invasive strains was not obvious for the examined W:nt:P1.18-1,3 isolates, but the NZ04/140 disease-associated isolate was an outlier with very poor survival and there were only two carriage isolates for comparison. Although the differences in survival between the B, C and W135 strains might be related to capsule structure, survival differences between carriage and invasive isolates is likely dependent at least in part on other factors. The N. meningitidis genome is highly variable and has been cited as a paradigm for organisms that use genome variability as an adaptation to environmental challenge [Reference Schoen30, Reference Joseph31]. Multiple genes undergo phase variation (heritable but reversible switching on or off, to evade the immune system) and strains that are identical by laboratory typing are nevertheless likely to differ extensively in terms of total expressed proteins [Reference Seifert32, Reference Omer33]. In addition, Neisseria spp genes have been shown to undergo DNA rearrangement during host infection and are modulated in expression in response to host temperature [Reference Segal34, Reference Loh35].
Tzeng et al. reported that environmental survival of desiccated N. meningitidis did not differ between encapsulated and isogenic non-encapsulated strains, suggesting for this comparison that the capsule did not have an important role in survival [Reference Tzeng, Martin and Stephens18]. Our analysis of the C:2a:P1.7-2,4 and W:2a:P1.7-2,4 isolates, that are known to differ in capsule polysaccharides and in expression of a polysialyltransferase and an O-acetyl transferase that affect capsule composition [Reference Beddek19], provided an opportunity to examine possible effects of the capsule on environmental survival. Although the differences in survival represented by the
![]() $\hat \beta $
values did not reach significance (P = 0·077), the C isolates had higher initial survival and great maximum survival times. It is therefore possible that capsule structure may have an effect on resistance to desiccation and environmental survival, notwithstanding the caution noted above that other genetic variation may have contributed to the differential survival.
$\hat \beta $
values did not reach significance (P = 0·077), the C isolates had higher initial survival and great maximum survival times. It is therefore possible that capsule structure may have an effect on resistance to desiccation and environmental survival, notwithstanding the caution noted above that other genetic variation may have contributed to the differential survival.
Survival and the potential for transmission off fomites has been reported for other bacteria, including the gram-positive Streptococcus pneumoniae, which like N. meningitidis colonizes the nasopharynx and is generally believed to be transmitted by contact from respiratory secretions [Reference Walsh and Camilli36]. Although the presence or absence of a capsule had little effect on survival after drying for S. pneumoniae, there is evidence that the capsule can affect survival after drying in some bacteria. Mordhurst et al. found that the O-acetylation of Escherichia coli K1 strain capsule sialic acid by a polysialic acid O-acetyltransferase gave increased resistance to desiccation [Reference Mordhorst37]. The capsule sialic acid of N. meningitidis C, W135 and Y, but not B, serogroups is also O-acetylated, with acetylation thought to decrease immunoreactivity and to increase virulence through masking of the capsule polysaccharide epitope from the immune system [Reference Claus38].
Implications for survival and transmission
The survival of N. meningitidis on fomites indicates that it should be considered with other bacteria (including pathogens) that persist on surfaces. In our experiments, survival of some strains of dried meningococci was greater at 22% RH compared with 30% RH at 30 °C, with minimal (<1 h) survival after incubation at 36 °C and 95% RH. The 22% and 30% RH conditions we examined did not span the 28%–64% ambient RH range in the laboratory during our experiments, but the results indicate that humidity does affect survival of some N. meningitidis strains on fomites. These results are similar to those for other bacteria where there are effects of temperature and humidity on survival with possible implications for transmission from fomites [Reference McEldowney and Fletcher26–Reference Lopez29, Reference Kramer, Schwebke and Kampf39]. Although we did not set out to examine survival at the temperature and humidity combinations recorded in the laboratory, it is possible that the greater survival of NZ98/254 we observed in winter (Fig. 2) is related to the climatic conditions including lower humidity. We also note that the incidence of meningococcal disease in New Zealand is greatest during winter and spring [Reference Martin, Lopez and Sherwood25] although there is not as yet any evidence that transmission from fomites contributes to this pattern of disease.
Two other observations may be important for understanding the pathogenesis of meningococcal disease. First, the relatively prolonged survival of the Cuban, New Zealand and Norwegian epidemic isolates might be related to the virulence of these organisms and their capacity to cause prolonged epidemics. Second, the greater persistence of carriage compared with invasive isolates might reflect adaptations that confer survival outside the host or in the nasopharynx. It is of course possible that advantages that enhance environmental survival also impact interactions with the host during infection, carriage or invasion.
Overall, the current work identifies environmental factors that may promote survival and transmission of meningococci outside the host. Further work is required to examine differences in survival among carriage and invasive strains from a range of serogroups and to characterize the molecular mechanisms that underlie survival. The results also support the relevance of public health warnings against sharing drinking vessels in community situations.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268817002473.
ACKNOWLEDGEMENTS
This PhD research of CLS was funded by an ESR PhD scholarship. We are grateful to Chris Sissons and Lisa Wong of the Dental Research Unit, Wellington School of Medicine, for the gift of Defined Medium Mucin.
DECLARATION OF INTEREST
The authors declare no conflict of interest.









