INTRODUCTION
Norovirus (NoV) is one of the most common causes of sporadic and outbreak-associated gastroenteritis, particularly in the developed world [Reference Lopman1–Reference Patel4]. Most reported outbreaks occur where individuals live in close proximity to each other, with prolonged NoV outbreaks frequently reported in individuals who reside and work in long-term care facilities (LTCFs) [Reference Blanton5–Reference Rosenthal7].
Transmission of NoV can occur through the faecal–oral route, through ingesting particles of vomitus that have been aerosolized, and through contact with contaminated environmental surfaces [Reference Glass, Parashar and Estes8]. Symptoms include rapid onset of severe vomiting and diarrhoea, and can occur with little or no prodrome. The low inoculums (⩾18 viral particles) [Reference Teunis9] required for transmission and prolonged period [Reference Rockx10] of faecal shedding [Reference Atmar and Estes11] make the spread of NoV infections difficult to control.
NoV-associated outbreaks involving multiple LTCFs have been frequently reported. Possible relationships to explain occurrences of simultaneous multi-facility outbreaks have included staff members who worked or had social interactions between multiple affected facilities [Reference Calderon-Margalit12, Reference Yamagami and Hara13], transfers of infected patients [Reference Miller14], foodborne transmission [Reference Ohwaki15], and illness following a pilgrimage [Reference Fretz16]. Large community outbreaks are often the results of NoV-contaminated water [Reference Yoder17], and thus such outbreaks would not be confined to these settings. Overall, attempts to find associations between simultaneous NoV outbreaks have been challenging due to the many institutions involved, numerous people affected, and complex resident and staff interactions.
Here, we describe the epidemiology of suspected transmission of NoV in eight long-term care facilities in Clark County, Nevada, USA, during February–March 2010. Clark County has a land mass of 7910 square miles (20 487 km2) and its 1·9 million residents make up nearly 75% of the state's population. The county encompasses five incorporated cities including Las Vegas, the site of the majority of these outbreaks. We will also show the methods used to identify the sources of the infections, the detection of a concurrent outbreak of Clostridium difficile, and the infection control measures used to control the NoV outbreaks.
METHODS
Epidemiological investigation
The Southern Nevada Health District (SNHD) received the first acute gastroenteritis (AGE) cluster report at a LTCF in Clark County in February 2010. In the month that followed, reports regarding AGE illnesses in seven additional LTCFs were received. We conducted telephone interviews with the reporter from each facility for the following information: the census of residents and staff, illness onset date for case(s), illness symptoms and duration, age of ill people, preliminary infection control measures already instituted by the LTCFs, and hospitalized persons and deaths. Names of hospitalized individuals were cross-matched with all deaths in Clark County 3 months after the outbreaks to determine if any had died. The identity of patients who were ill with AGE symptoms shortly before they were transferred into an affected LTCF was also requested.
Mantel–Haenszel χ2 tests were used to compare proportions of hospitalization and attack rates in the facilities that provided 24-h nursing care to residents to the ones that did not. Fisher's exact two-tailed P value was reported when a sample size was ⩽5. P values ⩽0·05 were considered significant.
We also performed an early assessment of the symptoms experienced by ill people to narrow the possible pathological agents and investigated the possible associations between these LTCFs.
Case-finding
A case was defined as a LTCF resident or staff who, as reported by the facility's administrator, experienced at least ⩾3 loose stools and/or ⩾1 episodes of vomiting in a 24-h period.
The SNHD database of reported outbreaks was searched for all AGE outbreaks between January and April 2010, the period encompassing the month prior to and the month after the conclusion of these eight outbreaks. The number of licensed LTCFs and hospitals in Southern Nevada was obtained from the Nevada State Health Division.
To identify staff members who worked at multiple LTCFs, the staff rosters and job descriptions from all affected facilities were obtained and names were cross-matched. Staff illness timelines were confirmed with staff's employers or State investigators.
Illness in food-handlers (staff members whose job titles indicate they may prepare or serve food in the facility's kitchen and dining room, and does not include staff who may feed residents), food catering companies, and grocery stores where food might be purchased were also obtained from affected LTCFs.
Laboratory testing
The respective staff of facilities C and F selected recently ill cases and collected their stool specimens (n=9), which were processed by the Southern Nevada Public Health Laboratory (SNPHL). Real-time reverse transcriptase–polymerase chain reaction (rRT–PCR) testing for NoV, enzyme-linked immunosorbent assay for rotavirus, and bacterial cultures (Salmonella, Shigella, Campylobacter, strain O157 of Escherichia coli, Yersinia) were performed on these stool samples. NoV-positive specimens were forwarded to the Nevada State Public Health Laboratory for sequence typing and genetic analysis.
Facilities B and E submitted their respective stool specimens from ill persons to the same local commercial diagnostic laboratory. Facility C subscribed to a second local laboratory that was different than facilities B and E. We compared medical records reviews for laboratory submissions from these three facilities to the two commercial laboratories concerning four tests [NoV, C. difficile, ova and parasites (O&P), and cultures for enteric pathogens] for February–March 2010. Only the first laboratory submission from each person for each test was counted. These commercial laboratories employed rRT–PCR to detect NoV in four stool specimens and the rest by enzyme immunoassay (EIA), antigens to detect C. difficile toxins A and B by EIA, microscopic evaluations for O&P, and cultures for bacterial pathogens. Four facilities submitted no stool specimens.
RESULTS
Outbreak investigation
Of 1797 persons who resided or worked at the eight LTCFs, 394 (22%) met the case definition (Table 1). Of 954 residents, 299 (31%) were ill, and of 843 staff, 95 (11%) were ill. Symptoms were first observed in staff at three facilities, with subsequent spread to other residents and staff. The outbreaks began during a 4-week period, with 11 days (range 5–33) the median duration of each outbreak. The epidemic curve summarizes the outbreaks and shows the starts of the outbreaks were staggered (Fig. 1).
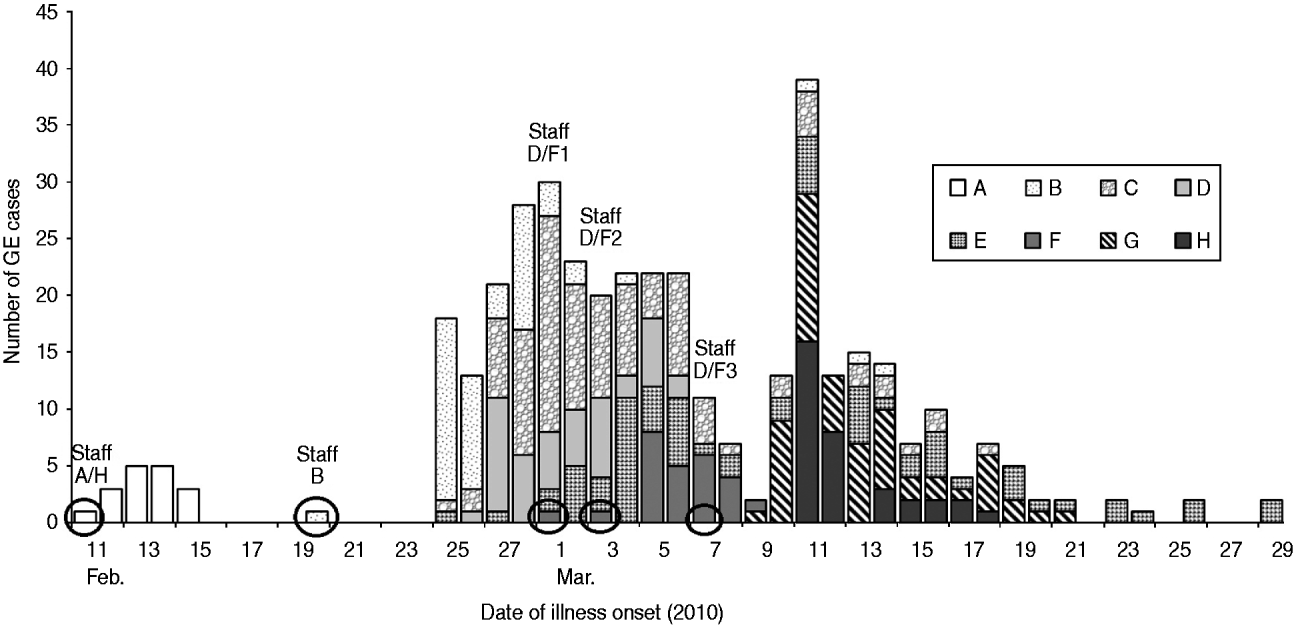
Fig. 1. Distribution of cases by illness onset date (n=394) at eight long-term care facilities, Clark County, Nevada, USA, February–March 2010. Circled areas indicate onset date of a staff member.
Table 1. Summary of residents and staff affected, and distribution of laboratory tests submitted, at eight long-term care facilities, Clark County, Nevada, USA, February–March 2010
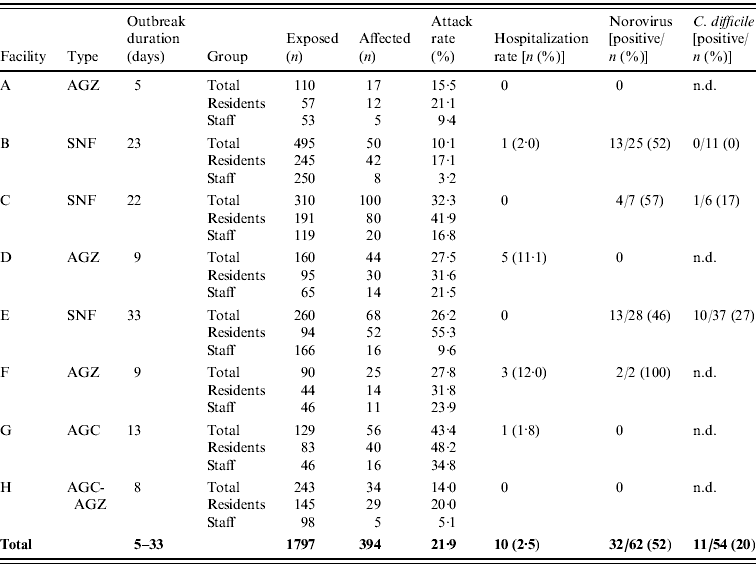
AGZ, Adult Group Care for Alzheimer's; SNF, Skilled Nursing Facilities; AGC, Adult Group Care; n.d., no data (medical records reviews for laboratory submissions were not performed).
Table 1 shows three were licensed as Skilled Nursing Facilities (SNF), which were mandated to provide 24-h care to residents by a skilled on-site nursing staff. Five were residential care facilities [one Adult Group Care (AGC), three AGCs for Alzheimer's (AGZ), and one AGC-AGZ] which were not required to provide 24-h nursing care to residents. Available resident beds were 75–97% filled to capacity at all except at facility A (38%). Five affected facilities were located in Las Vegas and three in the adjacent city of Henderson, and facilities were separated between 1·5 miles (2·4 km) and 27·6 miles (44·4 km).
Attack rates were higher in residents (range 17–55%) than staff (range 3–35%) in all facilities. The median number of ill residents and staff in all eight LTCFs was 35 and 12·5 persons, respectively. Attack rates between the AGCs, the AGZs, and the SNFs were not significantly different from each other.
Affected staff (n=85, age range 19–78 years, median 43·5 years) were comparatively younger than affected residents (n=225, age range 44–99 years, median 84·5 years).
Of 207 cases, 176 (85%, range 68–100%) experienced diarrhoea and 98 (47%, range 19–64%) vomiting. Reporting of symptoms was performed by all three SNFs, but only in two of the five AGCs and AGZs. Illness duration characteristically lasted 24–48 h and was self-limiting in non-hospitalized persons. Ten affected residents and no staff were hospitalized (Table 1). Hospitalization rates were significantly higher in the AGCs and AGZs, compared with the SNFs (ranges 0–12% vs. 0–2%, respectively, Fisher's exact P=0·0063). Ill residents received varying levels of hydration therapy at all facilities, and SNF residents who tested positive for C. difficile were treated with antibiotics at their respective facilities by their physicians. No fatalities occurred.
The epidemic curves (Fig. 1) suggest that transmission occurred by person-to-person contact at these facilities. The high number of ill persons reported on the first day at facility H was due to delayed recognition of the outbreak by administrators, as its AGC and AGZ units were located in separate buildings.
Since NoV was detected at all facilities where testing occurred, outbreaks at facilities where there were no microbial evaluation and outbreak characteristics did not meet the Kaplan criteria [Reference Kaplan18] were also suspected to be NoV infections. All LTCFs were thus instructed to implement NoV infection control measures in healthcare settings based on recommendations by the Centers for Disease Control and Prevention (CDC) [19], with ill staff excluded from work for 72 h after resolution of symptoms, hand-washing with soap and water, and intensive environmental cleaning with bleach or products effective against feline caliciviruses from an environmental protection agency-approved list (http://www.epa.gov/oppad001/list_g_NoV.pdf).
Case-finding
Outbreak duration (Table 1) at each LTCF began from the day facility administrators observed cases until there were no new cases. Each facility was surveyed until no case was reported for seven consecutive days. The distribution of cases for all affected facilities over time is illustrated in Figure 1. Illnesses from seven facilities were reported by their respective administrators, and illness at facility E was reported by an administrator of facility D who noticed there were many ill people at the former facility. Facility E identified C. difficile infections during the course of its NoV outbreak. Prior to reporting the outbreaks, 80 (20%) of the cases had already been noted by the facilities' administrators, with the remaining cases (n=314, 80%) reported to us during active surveillance.
No other AGE outbreak was reported between January and April 2010 in the 28 SNFs, 147 AGCs, and 84 AGZs facilities licensed in Southern Nevada. An AGE outbreak (3–17 March 2010) was reported at one of the 29 hospitals in Southern Nevada. This hospital was excluded from analyses because it was not a LTCF, did not admit hospitalized residents from affected facilities, only staff were ill, and no ill staff worked at multiple facilities.
Staff frequently moved back and forth between the affected facilities. Cross-referencing the names on the employee rosters from all facilities revealed nine staff members who worked or had social interactions in several facilities. Of these, eight (four ill) were identified as being employed in multiple affected facilities: facilities A and D shared one staff (none ill), A and H shared two (one ill), B and H shared one (none ill), C and E shared one (none ill), and D and F shared three (all ill). Staff connection between facility G and the other affected facilities was not identified. At least three outbreaks were preceded by illness in these staff members. The ill employee who worked at facilities A and H may have served as the source case for facility A (staff A/H, Fig. 1). Two of the three shared workers from facilities D and F became ill shortly prior to the outbreak at facility F, and may have served as source cases (staff D/F 1–2, Fig. 1). Along with the third shared employee (staff D/F 3, Fig. 1), all three workers were included among ill people of facility F. The ninth staff member was suspected of being the source case at facility B (staff B, Fig. 1) and was not employed at more than one facility. However, this worker became symptomatic after she visited her parent, who was ill with AGE symptoms and received care at the parent's home from a hospice company that also provided services to facility D.
None of these outbreaks was preceded by illness in kitchen and food service staff. These facilities shared two large food catering companies, which also distribute food to numerous non-affected LTCFs and local restaurants. Some facilities periodically purchased food from local chain grocery stores, of which none were common.
All facilities routinely accepted new residents from private homes, hospitals, and other LTCFs into their facilities prior to their respective outbreaks. Cessation of new admissions occurred at all but one facility during the course of their respective outbreaks. Facility E, however, continued to admit new residents into its facility after the start of its outbreak. Four residents were noted as ill with gastroenteritis symptoms upon admittance into facility E during its outbreak period, with one of these residents an arrival from facility D.
Laboratory testing
Of 62 stool samples submitted, 32 (52%) were positive for NoV (Table 1). The 172-nucleotide (nt)-long B-region PCR products of the NoV genome were obtained from four of six specimens, with two rejected for analyses due to low viral load. Three NoV sequences from facility C appeared to be identical, with 99% nt identity (three nt substitutions) to NoV strain Hu/GII.4/Orange/NSW001P/2008/AU [GenBank accession no. GQ845367; and CDC CaliciNet Database (Atlanta, USA) reference GII.4 New Orleans (NSW001P_AUS08)]. The sequence submitted by facility F differed from facility C's sequences by 1 nt. Sequences obtained in this study were submitted to Genbank and were assigned accession numbers JN049637–JN049640.
Table 1 shows that of 54 stool specimens tested for C. difficile, 11 (20%) were positive: ten came from facility E and one from facility C. Two of the positive C. difficile specimens from facility E were obtained from ill residents whose stool specimens were also positive for NoV. No specimen submitted by facility B was positive for C. difficile.
All 31 stool specimens submitted for O&P, 17 for cultures for enteric pathogens, and seven for rotavirus testing were negative (data not shown).
DISCUSSION
These AGE outbreaks affected eight LTCF institutions with 394 identified cases in a 4-week period. Cases had similar symptoms, and almost identical NoV sequences were detected from cases at two affected facilities. The simultaneous detection of NoV and C. difficile in residents is indicative of co-infections in at least one facility. Ten residents were hospitalized, and no death occurred.
Since GII.4 strains of NoV are frequently transmitted by person-to-person spread in closed or semi-closed settings [Reference Friesema20–Reference Kroneman22], this most likely accounted for the propagated mode of transmission of NoV in these facilities. As elderly residents seldom leave these facilities, this strongly implies that the initial transmission was from staff members to residents. In three facilities, the first known illness occurred in staff with subsequent illness in residents and staff. Exposure of care home staff to infectious work colleagues [Reference Vivancos23] as well as ill residents via direct contact with NoV-containing faecal matter or aerosolized vomitus, or by indirect contact with these via environmental surfaces may have spread the virus to other residents and staff in the course of their work [Reference Glass, Parashar and Estes8].
NoV sequences from single-source outbreaks are typically identical, but 1-nt difference in NoV sequences during prolonged outbreaks have been noted [Reference Lopman24]. The homology between the NoV sequences obtained in these outbreaks is very high compared to the 90% sequence identity (about 21 nt difference in viruses) reported previously in a multi-LTCF outbreak involving six nursing homes [Reference Calderon-Margalit12]. Furthermore, workers who had interactions with multiple homes did not develop symptoms, which could explain the spread of NoV between those homes. Although the relatedness of the outbreaks cannot be based solely on molecular evidence given that NoV GII.4 New Orleans has been circulating widely since 2009 throughout the USA [Reference Zheng25], the isolation of almost identical NoV genetic sequences from different facilities, the short time period when all the outbreaks occurred, and evidence that staff frequently moved back and forth between facilities provide strong foundations for the conclusion that illness in these eight LTCFs was linked.
The median duration of these outbreaks (11 days, range 5–33 days) is similar to other reported NoV outbreaks in LTCFs (10 days, range 7–14 days) [Reference Kirk26] and (12 days, range 2–35 days) [27]. The proportion of cases with vomiting (47%) was lower than those reported previously in LTCFs [Reference Rosenthal7, Reference Friesema20, Reference Kirk26]. Incomplete reporting of clinical data by some affected facilities may have contributed to these differences.
The consequences of NoV infection in elderly populations, who often have underlying medical conditions, can be severe resulting in hospitalization and death [27, Reference Harris28]. Observed case-hospitalization and death rates were 2·5% and 0%, respectively, which are comparable to those observed in LTCFs in Oregon, USA (3·1% and 0·5%, respectively) [Reference Rosenthal7]. However, these rates were different than those reported in nursing homes in Israel (10·2% and 2·0%, respectively) [Reference Calderon-Margalit12] and The Netherlands (0·5% and 1·6%, respectively) [Reference Friesema20]. These differences may simply reflect differences in the study populations, as even our analyses showed significant differences in hospitalization rates in the LTCFs that provided 24-h nursing care to their residents compared to those that did not. The disparity in the different types of LTCFs and its influence on NoV hospitalization and mortality rates in elderly residents requires closer examination.
The positive C. difficile laboratory results highlight the importance of laboratory testing for multiple potential pathogens during an outbreak. Infections of C. difficile have been detected in AGE outbreaks at healthcare institutions when the true aetiological pathogen was NoV [Reference Barrett29, Reference Koo30]. However, we do not believe that detection of C. difficile colonization was due to increased testing [Reference Martin31, Reference Wilcox and Fawley32] or false-positive results associated with these tests [Reference Bignardi, Staples and Majmudar33, Reference Svraka34] during AGE outbreak at LTCFs, because 91% of positive C. difficile tests were from one facility. Additionally, poor adherence to strict facility isolation during an outbreak could have led this facility to incur concurrent infections by C. difficile and NoV, as residents with gastroenteritis symptoms were still being admitted after its outbreak had begun. The facility's outbreak timeline suggests the C. difficile infections may have occurred prior to and independently of NoV, and both pathogens were uncovered due to the NoV investigation. Prompt and meticulous enforcement of guidelines to control NoV in LTCFs [Reference Friesema35, Reference Said, Perl and Sears36], and aggressive isolation intervention measures during an outbreak may be necessary to limit the introduction of new pathogens in these settings.
Finally, staff members who were simultaneously employed at multiple LTCFs can facilitate the spread of NoV between them. Because of the difficulty in identifying such shared employees, it is imperative to stress to LTCF administrators that all ill staff, in addition to being excluded from work for up to 72 h after the cessation of symptoms [Reference Atmar and Estes11, Reference Vivancos23, 37], should also not work at other LTCFs within this period. It is estimated that 32% of individuals who are infected with NoV may be asymptomatic [Reference Graham38]; it was thus possible that additional infected residents and staff were undiagnosed. Of the symptomatic staff and residents, underreporting may occur more in staff. For example, repeated bouts of diarrhoea or vomiting in elderly residents may be noticed and recorded by staff; however, staff members can work while ill or fail to report their illnesses to employers. Regulatory agencies should consider mandating ill staff who work at multiple facilities to observe work exclusion guidelines at all places of employment during NoV outbreaks.
There are several limitations of our investigation. One constraint was limited laboratory resources prevented documentation of aetiology at all facilities. All affected facilities were solicited to test ill persons; however, only half of the facilities complied and stool collection occurred mainly at the SNFs. This suggests that public health investigators need to supply assistance to LTCFs, especially ones without a 24-h skilled nursing staff, to ensure clinical specimens will be properly collected, tested, and the genetic sequences determined by sequencing during NoV outbreaks. Second, our surveillance of NoV and other AGE outbreaks in these settings have relied mainly on mandatory reporting by LTCF administrators, laboratory directors, emergency rescue personnel, and members of the public. This passive reporting process does not have the sensitivity to monitor for the complete reporting of illnesses related to such large outbreaks. For example, we were unable to establish if transmission resulted in cases in non-LTCF staff (e.g. employees of hospice programmes), who may also travel between LTCFs and have contact with residents during the course of their work. A more responsive monitoring system to detect NoV outbreaks can provide additional evidence towards the different methods of NoV transmission to better target infection control efforts.
Once recognized, the public health investigation led to the rapid identification of these multi-facility NoV outbreaks. Findings from the epidemiological investigation underscore the importance of diagnostic testing of ill persons; such testing discovered a concurrent outbreak of C. difficile that might otherwise have gone undetected. LTCFs need to adhere to national guidelines for the control of NoV and other nosocomial infections, and to take measures during an outbreak to isolate the affected from the unaffected. Special efforts need to be taken to identify staff members who are employed or have interactions with multiple facilities.
ACKNOWLEDGEMENTS
We gratefully acknowledge the following individuals for their contributions to the investigation and for their continuing collaboration: Southern Nevada Health District, Office of Epidemiology staff for epidemiological and disease investigations: Devin Barrett, Tony Fredrick, Jennifer Harmon, Jonathan Hyatt, Brian Labus, Zuwen Qiu-Shultz, Patricia Rowley, Mu Mu Tha, and Linda Verchick. The Southern Nevada Public Health Laboratory for collection of specimens and laboratory assistance: Pat Armour, Erin Buttery, and Sharon Johnson. The Nevada State Health Division for overseeing implementation of infection control measures: Ihsan Azzam, Francine Lincer, and Valle Oberg. The Nevada State Public Health Laboratory for genetic sequence typing and analysis: Sergey Morzunov.
DECLARATION OF INTEREST
None.




