INTRODUCTION
Tetanus is a severe, non-contagious infectious disease caused by Clostridium tetani, which is ubiquitous in the environment. Its spores are introduced through wounds contaminated with soil, dust or faeces. Other routes of infection include lacerations, burns or minor scratches; injection drug use and surgical procedures may be additional sources of infection [1]. Neonatal tetanus is a possible cause of mortality in the first month of life, especially in developing countries [Reference Bennett2].
Tetanus, Lyme disease, tick-borne encephalitis, hepatitis A, hepatitis E and anthrax are the most common biological risks for workers outside of healthcare units. Since 1963 (Law 292/63), vaccination against tetanus has been mandatory in Italy for professional categories such as farmers, metal workers, livestock breeders and waste workers as well as children during their second year (since 1968, vaccination has been required within the first year of age). Moreover, vaccination has been mandatory since 1938 for military personnel, explaining the higher incidence of tetanus among women in the past [Reference Filia3]. This is only mandatory vaccination for workers in Italy.
The tetanus vaccination schedule consists of a primary series (three doses) at the third, fifth and 11th months of age (mandatory), two boosters at 6 and 11–15 years of age, and one booster every 10 years (recommended) in the form of a combined tetanus–diphtheria (mandatory) or tetanus–diphtheria–acellular pertussis (recommended) vaccine. This strategy is similar to that adopted in other countries of the European Union and in the USA, and is generally considered to be highly effective in preventing tetanus, providing 95% protection to vaccine recipients [Reference Yildirim4].
The need for a decennial booster after five doses is a topic of debate, above all because the excessive use of boosters could result in severe side effects [Reference Edsall5] such as the Arthus phenomenon and allergic or systemic reactions. It is thought that these side effects are associated with the administration of a large number of doses over a short period of time, which was recommended practice in the 1950s; no instance of adverse effects has been published over the last 25 years [6].
The incidence of tetanus in Italy is lower than 1 case/1 000 000/year [Reference Filia3] but is higher than that reported in other European countries [7]. The incidence of tetanus infection is high in the elderly, particularly among elderly women [Reference Filia3].
The aim of the present study was to verify the persistence of protective antibody level after vaccination in order to determine whether the decennial booster is necessary. The influence of gender, age, the number of vaccine doses and the interval since the last dose on antibody titre was evaluated.
METHODS
Study design
According to the European Community (CEE directive 90/679) and Italian legislative decree 81/08, our unit submits all university workers and students to health surveillance. Among workers, subjects employed prevalently in the Agricultural, Engineering, Veterinary and Archaeology School of Padua University (1433 in total; 553 males and 880 females), as well as those in all departments with possible contact with soil, animals or waste who are required to be vaccinated, were examined between 2004 and 2011 for tetanus antibody titre according to health protocols established for our study.
The inclusion criteria were to have been born in Italy (to standardise the vaccination schedule) and to produce a booklet of vaccination released by a public health office.
Gender, age, the number of vaccine doses and the interval since the last dose were the independent variables considered in the study, whereas the antibody titre was the dependent variable.
All subjects agreed to the anonymous treatment of their personal data and signed a consent form. The research was based on data available due to health surveillance according to the law; the approval of the study by an ethics committee was not therefore required.
Analytical methods
The IgG-class of antibodies against Clostridium tetani toxin was measured in serum using the EIA (Enzyme Immune Assay) method (Radim, Rome, Italy). A tetanus antitoxin titre ⩾0·1 IU/mL is conventionally presumed to be protective (Centre for Disease Prevention and Control (CDC), 2006). In addition, a poor but sufficient protective level is defined to range from 0·11 to 0·5 IU/mL and a good protective level from 0·51 to <1 IU/mL; a long-lasting protective level is defined as ⩾1·0 IU/mL [Reference Chatchatee8]. This method does not measure values higher than 5 IU/ml, which is used as the upper bound.
Statistical analysis
Characteristics of the subjects and antibody titre were compared using parametric (unpaired t test) and non-parametric (Mann–Whitney) tests. The χ 2 test was applied to compare frequency distributions (Yates correction). Simple and multiple linear regression models and the prediction of multiple regressions were performed to identify associations between antibody titre (dependent variable) and age, gender, dose number and interval since the last dose (independent variables). The Cuzik trend test was utilised to evaluate the influence of dose number and interval since the last dose on the antibody titre. The subjects were categorised according to the number of doses (less than five, five and more than five) and the interval since the last dose (<5 years, 6–10 years, 11–15 years and >15 years). StatsDirect 2·7·7 (StatsDirect Ltd, UK) was used for all statistical analyses.
RESULTS
Subjects ranged in age from 18 to 64 years (Table 1), and the female subjects were on average significantly younger than males (P < 0·0001). Seventy-two of the subjects (5·0%) had an antibody titre below the supposed protective level of 0·1 IU/mL, while 254 (17·7%) appeared to be poorly protected. None of these subjects took immunosuppressive drugs. The majority (1107, 77·3%) had good (404, 28·2%) or long-lasting (703, 49·1%) protective titres. No difference was observed in antibody titre according to gender; thus, further evaluations were made without taking into account the gender of the subject.
Table 1. Age, number of vaccine doses, interval (years) since the last vaccine dose or booster, and antibody titre against tetanus toxin in the subjects enrolled in the study

The results are reported as the mean values ± s.d. (range) according to age, dose number and interval since the last dose; the median is given for antibody titre.
* Males vs. females: P < 0·0001.
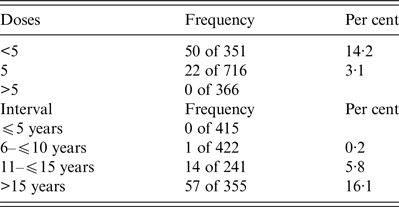
The majority of subjects (50%) completed the five-dose vaccination schedule, whereas 24·5% were vaccinated with fewer than five doses (range 3–4 doses) and 25·5% with more than five doses (range 6–9 doses). As expected, dose number significantly (P < 0·0001) influenced antibody titre (Fig. 1). In fact, a poor but protective titre (0·37 IU/mL, 0·05–0·96 on 355 subjects) persists for over 15 years (Fig. 2); in support of this, further analysis revealed that the majority of individuals with an interval longer than 10 years since the last dose displayed a protective titre unless they did not receive a booster (Table 2). Moreover, multiple linear regression predicted that five doses and 10 years since the last dose are predictive of an antibody titre of 1·99 IU/mL. Surprisingly, 4·5% of subjects meeting these criteria did not complete the primary vaccination schedule. Indeed, trend analysis confirms a significant influence of neither dose number nor interval since the last dose on antibody titre (P < 0·0001). The absence of protection (antibody titre below 0·1 U/ml) was consistently inversely correlated with both the number of doses and the time since the last dose (Table 3): 3·1% of individuals receiving five doses and 14·2% of those with fewer than five doses were not protected, and the number of non-protected individuals increased from 5·8% to 16·1% after 15 years. All but one of these subjects fall into the range time after last dose of more than 10 years. These data suggest that the majority of individuals that did not receive a booster 10 years after the last dose remained protected for at least 15 years. Using regression analyses, it was possible to establish a half-life of antibody titre of 10 years followed by a loss of the protective titre after 19·7 years. Finally, multivariate analysis suggests that gender could influence antibody titre (P = 0·0268), despite the absence of significance based on single variable comparison.

Fig. 1. Comparison among different doses of vaccine administered during the subjects’ lifetimes. All compared regimens exhibited a highly significant difference (P < 0·0001, two-sided, Mann–Whitney). Lines and diamonds in the box indicate medians, the edges of the boxes indicate quartiles, and the circles indicate fence values to define outliers for each dataset, as calculated by a statistics programme.

Fig. 2. Comparison among different intervals since the last vaccine dose. All compared intervals exhibited a highly significant difference (P < 0·0001, two sided, Mann–Whitney). Lines and diamonds in the box indicate medians, the edges of the boxes indicate quartiles, and the circles indicate fence values to define outliers for each dataset, as calculated by a statistics programme.
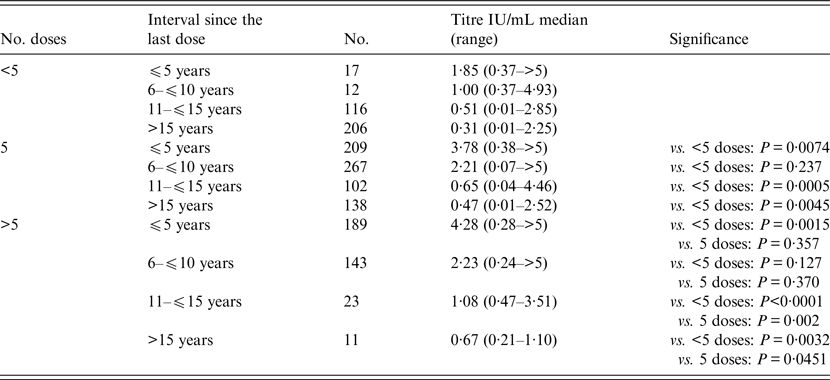
Table 2. Comparison of antibody titre according to number of vaccine doses and the interval since the last dose
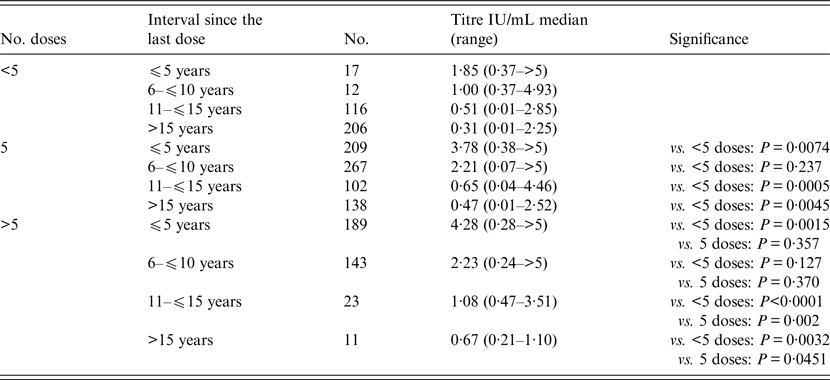
Statistical analysis was performed with non-parametric Mann–Whitney test.
Table 3. Frequency of subjects with a titre below the protective level (0·1 IU/mL), according to dose number (panel A) and interval since the last dose (panel B)
DISCUSSION
The goal of the present research was to investigate protection against tetanus among university workers exposed to this specific risk. In particular, we aimed to ascertain whether the decennial booster of tetanus vaccine recommended by CDC [9] and in Italy by the National Vaccine Plan 2016–2018 is necessary to maintain a protective antibody titre throughout life because it was demonstrated that immunological memory is persistent [10]. Among vaccine-preventable diseases, tetanus is infectious but not contagious; thus, vaccination does not contribute to herd immunity but rather is necessary as a personal preventive measure.
The results of the present study indicate that (i) after the primary vaccine series (three doses and two boosters at 6 and 11–15 years of age), the antibody titre persists at protective levels for a period in excess of 10 years and that (ii) vaccine doses influence antibody titre when categorised according to the interval since the last dose. For instance, we found that more than five doses guarantee a high antibody titre on average and that five vaccine doses are predictive of an antibody titre higher than 1 IU/mL at 15 or more years since the last dose. Indeed, according to our data, five doses are sufficient to maintain a protective titre for more than 15 years. This is consistent with the current knowledge that the titre half-life is 10 years and the drop to a non-protective level occurs after 20 years. We found that 11·9% of all individuals (71 of 596) aged >10 years had titres below levels considered protective. The percentage of non-protected individuals inversely correlates with the titre and directly correlates with the time since the last dose: 5·8% belong to the category included between 10 and 15 years, and 16·1% received the last dose more than 15 years ago. This indicates that the five-dose vaccination schedule works very well and maintains responsiveness for at least 15 years; thus, 1 or 2 boosters every 15 years could be sufficient to maintain a lifelong protective titre.
Several authors have correlated antibody titre with the age of the subject analysed. Alternatively, we suggest that the best parameter to predict tetanus reactivity is the time since the last dose when the vaccination schedule of five doses has been completed [Reference Coulibaly and De Serres11–Reference Peel14]. Other authors state that the reactogenicity of the tetanus vaccine is greater in young subjects and women [Reference Bayas15], while the elderly are highly seroprotected; thus, a single booster for secondary immunisation should be sufficient [Reference Hüllstrung16]. This is a different point of view that contrasts with the common opinion that advanced age increases risk [Reference Filia3]. Our results do not support an involvement of gender in the waning of antibodies against tetanus, but they definitely indicate that in the absence of boost after 10 years from the last dose, aged people became susceptible (non-protected) subjects.
Recently [Reference Hammarlund17], it has been suggested, according to the half-life of tetanus antibodies calculated at 14 years, that the decennial booster schedule should be changed to a schedule of two boosters delivered at 30 and 60 years of age. Our results indicate a half-life shorter than that estimated by these authors. Thus, we propose that countries with recommendations for decennial boosters should drop these in favour of longer intervals such as those now recommended in the UK, Australia and New Zealand. These findings confirm 50 years later both the observation of Edsall et al. [Reference Edsall5] in children and the theoretical approach of Gottlieb et al. [Reference Gottlieb18], establishing that approximately 55% of the population who have previously been immunised with tetanus vaccine and who have not received an intervening booster dose within the past 14 to 21 years will have a circulating antibody titre of 0·1 IU/mL or greater. In agreement with these positions, our report shows that a decennial booster should not be routinely administered because antibody titre persists at protective levels for a longer period, especially after five doses of the vaccine.
The limits of this evaluation are primarily environmental, since people with a waning titre may not have been exposed to tetanus toxin. Regarding the university workers, who are more likely to be exposed to tetanus, we recommend (i) to test whether antibodies have waned before giving a booster dose, provided that a rapid and affordable test becomes available [Reference Chithra19]; and (ii) to deliver a booster vaccination to those not protected, those who received the last dose at least 15 years ago, and those coming from extra-European countries where the vaccination plan is not implemented or the vaccination coverage is poor.
Declaration of Interest
None.