No CrossRef data available.
Published online by Cambridge University Press: 01 September 2022
Antidepressants that offer a rapid onset of action without requiring chronic use are greatly needed in both major depressive disorder (MDD) and postpartum depression (PPD). Zuranolone is an investigational, oral, neuroactive steroid and GABAA receptor positive allosteric modulator in clinical development as a 2-week treatment course for MDD and PPD.
To present the efficacy and safety of zuranolone vs placebo in Phase 2 and 3 trials.
In the studies presented (Table 1), improvements in depressive symptoms were assessed by least-squares mean (LSM) using a mixed-effects model for repeated measures on the change from baseline (CFB) at Day 15 in the 17-item Hamilton Rating Scale for Depression total score (HAMD-17; primary endpoint for all trials) and the Montgomery–Åsberg Depression Rating Scale (MADRS; secondary endpoint) following a 14-day treatment course of once-daily zuranolone.
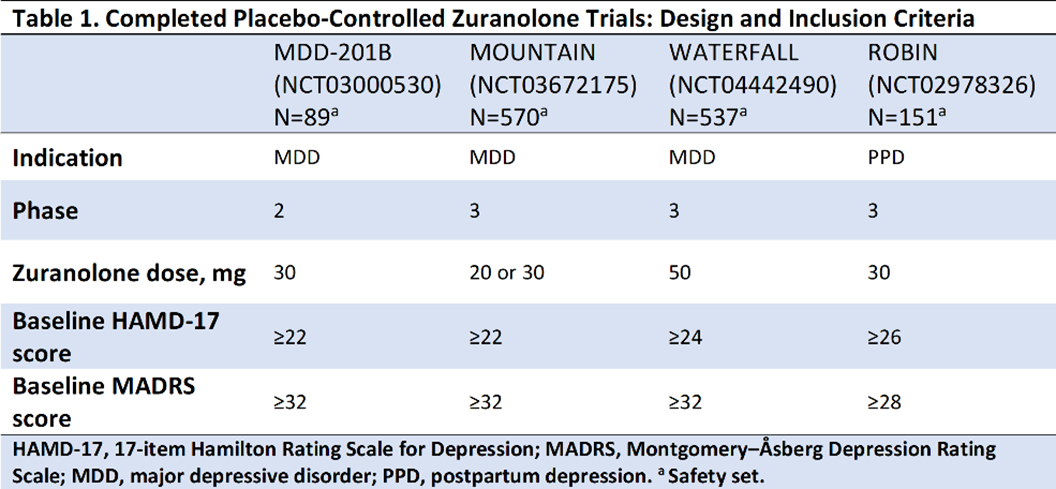
Compared with placebo, zuranolone treatment led to rapid improvements in depressive symptoms across clinical trials, with significant improvements (LSM treatment difference [SE] in CFB) in HAMD-17 and MADRS scores at Day 15 in 3 of the 4 trials (Table 2). Common treatment-emergent adverse events (≥5% in zuranolone treatment arms) were headache, somnolence, dizziness, nausea, sedation, diarrhea, upper respiratory tract infection, and fatigue (Table 3). No incidences of loss of consciousness or excessive sedation were observed.
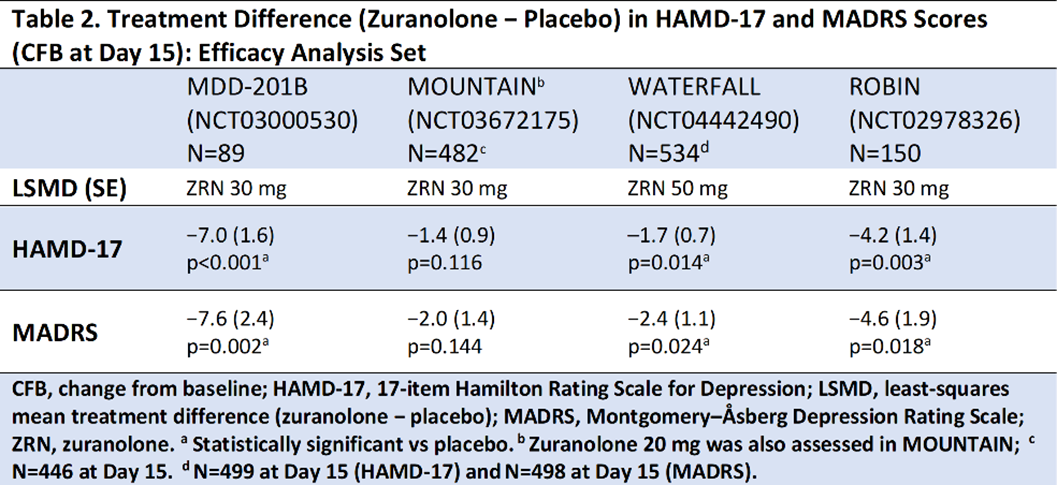
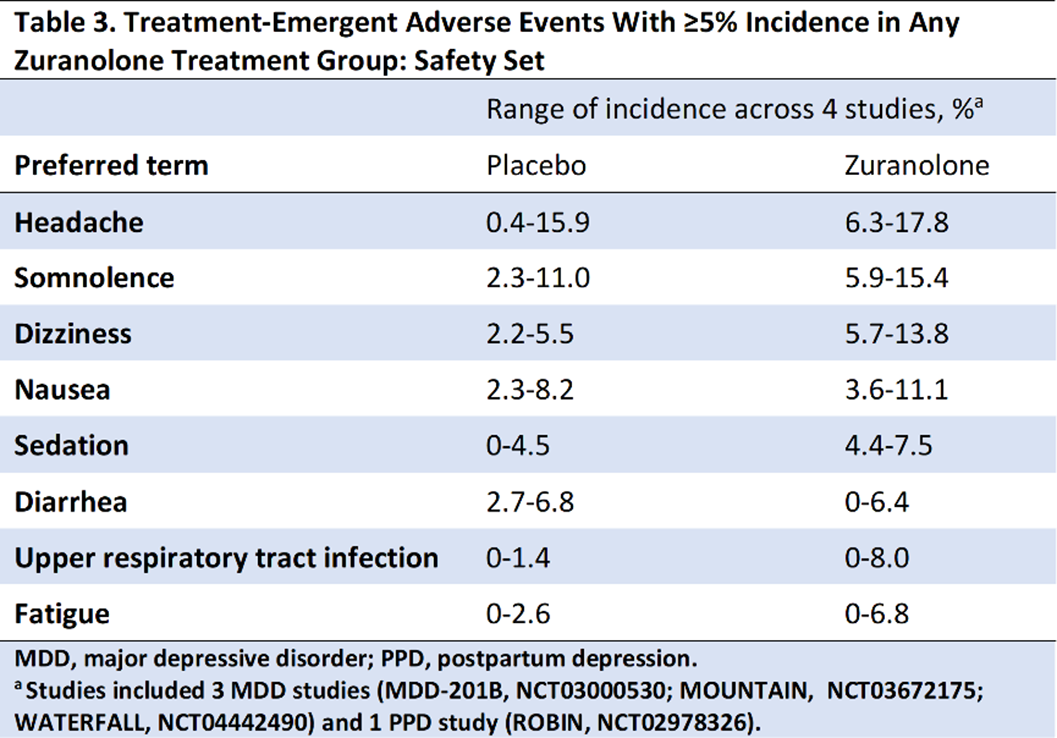
Across the completed studies in the zuranolone clinical trial program, patients receiving zuranolone consistently experienced improvement in depressive symptoms following a 2-week treatment course. Treatment with zuranolone was generally well tolerated with a consistent safety and tolerability profile.
The MDD-201B, MOUNTAIN, and ROBIN studies were sponsored by Sage Therapeutics, Inc; the WATERFALL study was sponsored by Sage Therapeutics, Inc, and Biogen. Medical writing and editorial support were provided by MediTech Media, Ltd, and funded by Biogen.
Comments
No Comments have been published for this article.