To the Editor—Blood culture remains the gold standard for the diagnosis of bloodstream infections. In most settings, only 5%–13% of blood cultures are positive, and 20%–56% of those represent contaminants.Reference Dargere, Parienti and Roupie 1 , Reference Lamy, Dargere, Arendrup, Parienti and Tattevin 2 Historically, blood culture contamination rates in our university-affiliated hospital have been consistently greater than the international recommended rate of 3%.Reference Hall and Lyman 3 If early clinical decisions are based on the contaminants, patients may be subjected to needless risk, and subsequently, health boards can be subjected to additional and unnecessary costs.Reference Alahmadi, Aldeyab and McElnay 4 Therefore, we developed a quality control circle, following the plan–do–check–act (PDCA) process, with an interdisciplinary task force to reduce the blood culture contamination rate. The objective of this study was to evaluate the effectiveness of a quality control circle in reducing blood culture contamination in our hospital.
A quality control circle group was established, and members were selected from the microbiology department, clinical wards, the nursing department, the administrative department, and the infection control department. Quality control circle members analyzed the causes of blood culture contamination related to the following categories: personnel, machine, material, method, and environment. Countermeasures were followed. The process of blood culture collection was standardized, and the use of the sterile procedure was advocated. In each clinical department ward, a venipuncture box was provided to hold a variety of equipment for blood sample collection. A comprehensive and continuous training program was held on schedule, including on-the-job training, drop-in educational sessions, and hands-on educational sessions. Contaminated cases were reviewed and discussed to determine which operator and/or patient factors were present. A WeChat work group was set up for timely release of information and communication of questions and answers (Q&A). A group meeting of the quality control circle was held monthly, and the group discussed continuous improvement measures regarding blood culture contamination. A feedback mechanism was also established, and the report was summarized and released monthly on the WeChat group.
As shown in Fig. 1, contamination rates of all the department wards, the mean contamination rates, and true-positive culture rates of the hospital were considered. In the prestudy period, the contamination rates of the divisions were high and varied from 6.1% to 10.3%. In total, 2,712 blood cultures were sampled, and 190 contaminated blood culture bottles were identified, giving a mean contamination rate of 7.0%, which is approximately double that of the international recommended rate. The true-positive rate of blood culture was 9.3% (253 of 2,712) during this period. After launching the quality control circle program, the monthly mean contamination rates decreased. At the end of the study period, the mean contamination rate was 2.3% (69 of 3,060), and the true-positive rate was 10.2% (312 of 3,060). Analysis of the data per division (Fig. 1) revealed a statistically significant contamination reductions in blood culture contamination in all categories. The mean contamination rate during the study period was significantly lower than that during the prestudy period (7.0% vs 2.3%; χ2 = 75.72; P<.001), while the true-positive rates did not change significantly (9.3% vs 10.2%; χ2 = 1.22; P = .27).
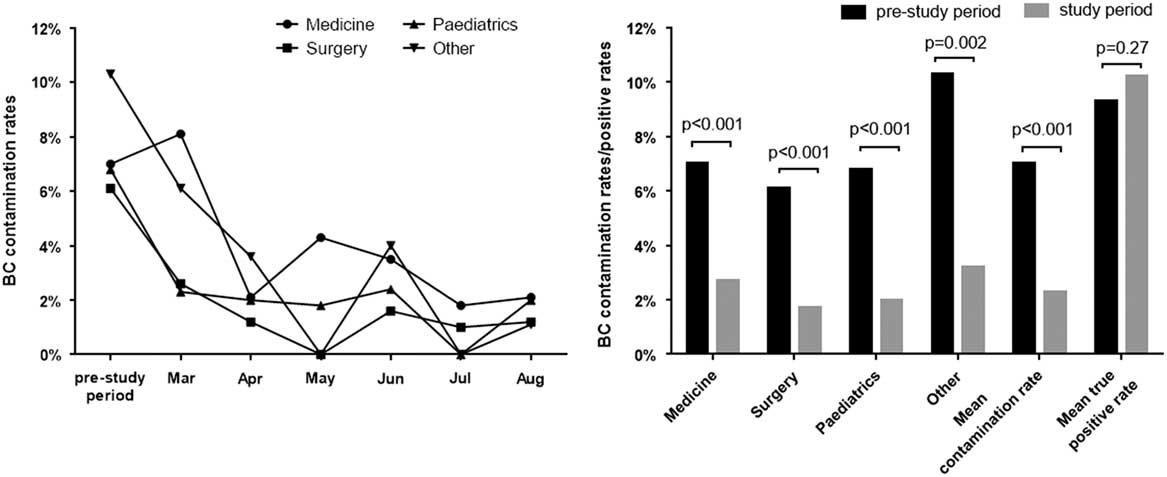
Fig. 1 Analysis of blood culture contaminated and positive rates for the prestudy period compared with the study period. A. Blood culture contaminated rates comparing monthly for the study period compared with the prestudy period. B. Proportions of blood culture contaminated and positive rates before and after implementing a quality control circle.
Several suggestions have been proposed for ways to reduce blood culture contamination rates.Reference Zimmerman, Assous, Yinnon and Wiener-Well 5 , Reference Self, Speroff and Grijalva 6 Recently, Zimmerman et alReference Zimmerman, Assous, Yinnon and Wiener-Well 5 suggested the use of a departmental report card to monitor the blood culture contamination rate; however, in some divisions, Zimmerman et al did not achieve a significant reduction in blood culture contamination rate. In another study, Self et alReference Self, Speroff and Grijalva 6 developed a sterile process for blood culture collection. A reduction in blood culture contamination rate was found, but only the emergency department and the blood culture collection process were evaluated. Some evidence also suggests that contaminants originate from patient skin flora and that up to 20% of skin flora may still be cultured on harvesting samples using sterile surgical techniques.Reference Hall and Lyman 3 Therefore, further work is needed to decrease blood culture contamination rates in more categories, and continuous improvement in the related processes is necessary. Here, the quality improvement project was a quality control circle. We selected effective countermeasures and achieved an encouraging result for reducing the blood culture contamination rates in this 6-month study.
Blood culture contamination is a significant problem faced by many hospitals. To effectively reduce blood culture contamination and to maintain the effectiveness of the processes implemented, cooperation between multiple departments and standardized procedures are needed. The quality control circle is a comprehensive and scientific management model, and it has been successful in reducing the blood culture contamination rates. After a circular plan–do–check–act process, the quality control circle project only needed minor revision. A sustainable reduction in contamination rates was then achieved.
Acknowledgments
Financial support
This study was funded by Chinese National Natural Science Foundation (No. 81470351), Foundation in the Fourth Round of the Shanghai Municipal Key Disciplines of Public Health on Transfusion Medicine (grant no. 15GWZK0501) and Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (grant no. PWRI2016-04). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



