Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Jaeger, Frederike
Matar, Omar K.
and
Müller, Erich A.
2018.
Bulk viscosity of molecular fluids.
The Journal of Chemical Physics,
Vol. 148,
Issue. 17,
Chen, Kang Ping
and
Shen, Di
2018.
Mechanism of fluid production from the nanopores of shale.
Mechanics Research Communications,
Vol. 88,
Issue. ,
p.
34.
Sharma, Bhanuday
and
Kumar, Rakesh
2019.
Bulk viscosity of dilute gases using non-equilibrium molecular dynamics approach.
Vol. 2132,
Issue. ,
p.
090003.
Sabatini, R.
Snively, J. B.
Hickey, M. P.
and
Garrison, J. L.
2019.
An analysis of the atmospheric propagation of underground-explosion-generated infrasonic waves based on the equations of fluid dynamics: Ground recordings.
The Journal of the Acoustical Society of America,
Vol. 146,
Issue. 6,
p.
4576.
Chen, Song
Wang, Xiaoning
Wang, Jianchun
Wan, Minping
Li, Hui
and
Chen, Shiyi
2019.
Effects of bulk viscosity on compressible homogeneous turbulence.
Physics of Fluids,
Vol. 31,
Issue. 8,
Sharma, Bhanuday
and
Kumar, Rakesh
2019.
Estimation of bulk viscosity of dilute gases using a nonequilibrium molecular dynamics approach.
Physical Review E,
Vol. 100,
Issue. 1,
Bhola, Sahil
and
Sengupta, Tapan K.
2019.
Roles of bulk viscosity on transonic shock-wave/boundary layer interaction.
Physics of Fluids,
Vol. 31,
Issue. 9,
Yuan, Zhenyu
Jiang, Zhongzheng
Zhao, Wenwen
and
Chen, Weifang
2020.
Multiple temperature model of nonlinear coupled constitutive relations for hypersonic diatomic gas flows.
AIP Advances,
Vol. 10,
Issue. 5,
Zheng, Qinmin
Wang, Jianchun
Noack, Bernd R.
Li, Hui
Wan, Minping
and
Chen, Shiyi
2020.
Vibrational relaxation in compressible isotropic turbulence with thermal nonequilibrium.
Physical Review Fluids,
Vol. 5,
Issue. 4,
Singh, Satyvir
Battiato, Marco
and
Myong, R. S.
2021.
Impact of bulk viscosity on flow morphology of shock-accelerated cylindrical light bubble in diatomic and polyatomic gases.
Physics of Fluids,
Vol. 33,
Issue. 6,
Yuan, Ze
Zheng, Qun
Yue, Guoqiang
and
Jiang, Yuting
2021.
Performance evaluation on radial turbines with potential working fluids for space closed Brayton cycle.
Energy Conversion and Management,
Vol. 243,
Issue. ,
p.
114368.
Ma, Qihan
Yang, Chunxin
Bruno, Domenico
and
Zhang, Jun
2021.
Molecular simulation of Rayleigh-Brillouin scattering in binary gas mixtures and extraction of the rotational relaxation numbers.
Physical Review E,
Vol. 104,
Issue. 3,
Kosyanchuk, Vasily
2022.
Effect of internal degrees of freedom in rarefied gas problems: Plane Couette flow.
International Journal of Heat and Mass Transfer,
Vol. 190,
Issue. ,
p.
122759.
Yuan, Ze
Zheng, Qun
Yue, Guoqiang
Zhang, Jie
and
Jiang, Yuting
2022.
Evaluation on the flow characteristics of helium working fluid in generation Ⅳ nuclear power station.
Nuclear Engineering and Design,
Vol. 389,
Issue. ,
p.
111667.
Yuan, Ze
Zheng, Qun
Jiang, Yuting
Jiang, Bin
and
Luo, Mingcong
2023.
Review on power conversion unit of noble gas closed Brayton cycle for space and underwater applications.
Applied Thermal Engineering,
Vol. 223,
Issue. ,
p.
119981.
Benhamou, Jaouad
Vincent, Bjarne
Miralles, Sophie
Jami, Mohammed
Henry, Daniel
Mezrhab, Ahmed
and
Botton, Valéry
2023.
Application of the lattice Boltzmann method to the study of ultrasound propagation and acoustic streaming in three-dimensional cavities: advantages and limitations.
Theoretical and Computational Fluid Dynamics,
Vol. 37,
Issue. 6,
p.
725.
Kumar, Anil
and
Rana, Anirudh Singh
2023.
H
-theorem and boundary conditions for two-temperature model: Application to wave propagation and heat transfer in polyatomic gases.
Physical Review E,
Vol. 108,
Issue. 6,
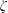 $\unicode[STIX]{x1D701}$ is derived by the continuum medium methodology, which provides a further understanding of the bulk viscosity, i.e.
$\unicode[STIX]{x1D701}$ is derived by the continuum medium methodology, which provides a further understanding of the bulk viscosity, i.e. 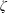 $\unicode[STIX]{x1D701}$ is equal to the product of the bulk modulus
$\unicode[STIX]{x1D701}$ is equal to the product of the bulk modulus 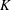 $K$ and the relaxation time
$K$ and the relaxation time  $\unicode[STIX]{x1D70F}$ (
$\unicode[STIX]{x1D70F}$ (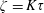 $\unicode[STIX]{x1D701}=K\unicode[STIX]{x1D70F}$). The continuum and kinetic theories present consistent results from macro- and microperspectives respectively, only differing in terms of a coefficient. The theoretical predictions of the BVC in diatomic molecules, such as
$\unicode[STIX]{x1D701}=K\unicode[STIX]{x1D70F}$). The continuum and kinetic theories present consistent results from macro- and microperspectives respectively, only differing in terms of a coefficient. The theoretical predictions of the BVC in diatomic molecules, such as  $\text{N}_{2}$,
$\text{N}_{2}$, 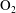 $\text{O}_{2}$ and CO, show good agreement with the experimental data over a wide range of temperature. In addition, the vibrational contributions to
$\text{O}_{2}$ and CO, show good agreement with the experimental data over a wide range of temperature. In addition, the vibrational contributions to 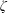 $\unicode[STIX]{x1D701}$ are controlled by a rapid exponential decrease at high temperatures, while at low temperatures a slow linear increase proceeds for the rotational cases. The relaxation time
$\unicode[STIX]{x1D701}$ are controlled by a rapid exponential decrease at high temperatures, while at low temperatures a slow linear increase proceeds for the rotational cases. The relaxation time  $\unicode[STIX]{x1D70F}$, collision number
$\unicode[STIX]{x1D70F}$, collision number 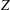 $Z$, BVC
$Z$, BVC 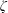 $\unicode[STIX]{x1D701}$ and ratio of bulk-to-shear viscosities
$\unicode[STIX]{x1D701}$ and ratio of bulk-to-shear viscosities  $\unicode[STIX]{x1D701}/\unicode[STIX]{x1D707}$ in the vibrational mode are found to be several orders of magnitude larger than those in the rotational mode.
$\unicode[STIX]{x1D701}/\unicode[STIX]{x1D707}$ in the vibrational mode are found to be several orders of magnitude larger than those in the rotational mode.


