Introduction
Genus Mesocestoides is controversial mainly because its systematics and life cycles are not completely understood (Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021). It is presumed to have a three-host life cycle, with carnivorous vertebrates, mostly mammals, as definitive hosts; meanwhile, coprophagous insects would act as first intermediate hosts, and small vertebrates, preying on these arthropods, as second intermediate hosts (Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005). Thus, the life cycle of Mesocestoides would be complete once the definitive host preys upon the secondary intermediate host (Padgett & Boyce Reference Padgett and Boyce2004). However, recently McAllister et al. (Reference McAllister, Tkach and Conn2018) suggested that there is a two-host life cycle based on the finding of pre-tetrathyridial larvae in the coelomic cavity of a ground skink (Scincella lateralis). Albeit there are myriad species recorded as intermediate hosts of Mesocestoides spp., its prevalence is frequently low (~7%) (Bursey et al. Reference Bursey, Goldberg, Telford and Vitt2012; Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021). Moreover, this tapeworm has been recorded in domestic dogs and cats as definitive hosts, and as accidental hosts harboring tetrathyridia in the peritoneal cavity, causing the so-called peritoneal larval cestodiasis, which could lead to a fatal outcome (Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021).
From the morphological point of view, the classification of Mesocestoides is controversial because measurements overlap, and morphological traits are similar in adult tapeworms of different species (Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005; Skirnisson et al. Reference Skirnisson, Jouet, Ferté and Nielsen2016a). Currently, only five of 28 described taxa have compelling morphological and molecular evidence and are considered as valid species: Mesocestoides litteratus, Mesocestoides lineatus, Mesocestoides melesi, Mesocestoides vogae (syn. Mesocestoides corti), and Mesocestoides canislagopodis (McAllister et al. Reference McAllister, Tkach and Conn2018; Berrilli & Simbula Reference Berrilli and Simbula2020; Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021). All the above-mentioned species have been recorded exclusively in the Holarctic realm (Berrilli & Simbula Reference Berrilli and Simbula2020). Meanwhile, in the Neotropical realm there are records of adult tapeworms parasitizing wild and domestic canids such as Mesocestoides variabilis from Peru (Angulo-Tisoc et al. Reference Angulo-Tisoc, Curie and Gomez-Puerta2020), Mesocestoides michaelseni from Brazil (Justo et al. Reference Justo, Fernandes, Cárdenas and Cohen2017), and “M. lineatus” from Argentina (Fugassa, Reference Fugassa2020) and Chile (Oyarzún-Ruiz et al. Reference Oyarzún-Ruiz, Di Cataldo, Cevidanes, Millán and González-Acuña2020). Also, larvae and adult worms of Mesocestoides sp. have been recorded from Argentina (González et al. 2013; Hamann et al. Reference Hamann, Kehr and González2014; Hamann & González Reference Hamann and González2015), Chile (Oyarzún-Ruiz et al. Reference Oyarzún-Ruiz, Di Cataldo, Cevidanes, Millán and González-Acuña2020), and Peru (Angulo-Tisoc et al. Reference Angulo-Tisoc, Curie and Gomez-Puerta2020). In addition, tetrathyridia have been found parasitizing lizards from Argentina (García et al. Reference García, Monachesi and Paz2015), Brazil (Justo et al. Reference Justo, Fernandes, Cárdenas and Cohen2017), Costa Rica, and Nicaragua (Bursey et al. Reference Bursey, Goldberg, Telford and Vitt2012). However, none of these studies supported the morphological diagnoses with genetic evidence. Therefore, there is an evident need to elucidate the life cycles of these tapeworms by applying an integrative taxonomic approach, i.e., combining morphological and molecular analyses to delimit species (Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018; McAllister et al. Reference McAllister, Tkach and Conn2018; Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021).
In Chile there are 131 species of reptiles, including genus Liolaemus (Liolaemidae) with 72 species, of which 57 are catalogued as endemic (Mella Reference Mella2017). The helminth parasites for this genus of lizard have been recorded in seven species. However, the identification to the species level of these parasites is not given (San-Martín-Órdenes et al. Reference San-Martín-Órdenes, Muñoz-Leal, Garín and González-Acuña2019). The present work aimed to include new data to the helminth fauna of native reptiles and to discuss, through a phylogenetic framework, the systematics of this intricated genus of tapeworms in the Neotropics.
Material and Methods
During March 2019, a total of 18 braided tree iguanas (Liolaemus platei), 8 females and 10 males, were collected in 2 sites from the Coquimbo region, northern Chile; Farellón Sánchez (31°26.8’S 71°1.1’O) and Cañas de Michío (31°38.2’S 71°3.8’O) (Supplementary Figure S1). The capture permit was provided by the state-owned organism Servicio Agrícola y Ganadero, Chile (334/2019). Lizards were captured with a sliding noose (Mella Reference Mella2017), kept inside individual cloth bags, and transported to the Laboratory of Ecological Interactions, Facultad de Ciencias, Universidad de Chile. Lizards were euthanized with an intracoelomic inoculation of 0.5% sodium thiopental (100 mg/kg). Parenchymatous organs and the coelomic cavity were dissected under the stereomicroscope for the presence of larval stages of tapeworms. Tapeworm larvae were washed in distillated water and fixed and preserved in ethanol 96%. Larvae were counted under a stereomicroscope, and different morphotypes were separated for morphological and molecular analyses. Larvae were stained with Carmine Alum, dehydrated, diaphanized, and mounted in Canada balsam following Lutz et al. (Reference Lutz, Tkach, Weckstein and Webster2017). Tetrathyridia were deposited in the collection of Museo de Zoología, Universidad de Concepción, under access number: MZUC-UCCC 47352.
Total genomic DNA was extracted from single worms for every morphotype by employing DNeasy blood and animal tissue kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions. DNA quantity and quality for each sample was tested with an EpochTM Microplate Spectrophotometer (Santodomingo et al. Reference Santodomingo, Robbiano, Thomas, Parragué-Migone, Cabello-Stom, Vera-Otarola, Valencia-Soto, Moreira-Arce, Moreno, Hidalgo-Hermoso and Muñoz-Leal2022). The extracted DNA was preserved at -24°C until analysis.
Conventional PCR (cPCR) was performed to amplify partial sequences of 18S rRNA and 12S rRNA loci, following Oyarzún-Ruiz et al. (Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). Primers and cPCR thermal conditions are provided in Supplementary Table S1. Amplicons of expected size were purified and sequenced in both directions at Macrogen (Seoul, South Korea). Sequences were verified for quality and edited with Geneious Prime® v.2021.2.2. (https://www.geneious.com). Basic local alignment searches were performed using the BLASTn tool (https://blast.ncbi.nlm.nih.gov), and similar sequences were downloaded from GenBank (https://www.ncbi.nlm.nih.gov). Alignments were constructed with MAFFT algorithm in Geneious Prime to then extract the informative regions, as suggested by Santodomingo et al. (Reference Santodomingo, Robbiano, Thomas, Parragué-Migone, Cabello-Stom, Vera-Otarola, Valencia-Soto, Moreira-Arce, Moreno, Hidalgo-Hermoso and Muñoz-Leal2022). The phylogenetic analyses for both loci were performed using maximum likelihood method (ML) and Bayesian inference (BI) with IQ-TREE v1.6.12 and MrBayes v3.2.6, respectively (see Oyarzún-Ruiz et al. Reference Oyarzún-Ruiz, Thomas, Santodomingo, Collado, Muñoz and Moreno2022). Consensus tree for ML and BI was generated for every locus following Santodomingo et al. (Reference Santodomingo, Robbiano, Thomas, Parragué-Migone, Cabello-Stom, Vera-Otarola, Valencia-Soto, Moreira-Arce, Moreno, Hidalgo-Hermoso and Muñoz-Leal2022).
Genetic pairwise distances between the sequences of the present study and sequences of valid Mesocestoides taxa (Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005; Berrilli & Simbul Reference Berrilli and Simbula2020; Jesudoss Chelladurai & Brewe Reference Jesudoss Chelladurai and Brewer2021) were computed using MEGA7 (Kumar et al. Reference Kumar, Stecher and Tamura2016). The sequences obtained in the present study were deposited in the NCBI GenBank database under the following access numbers: OQ701082-OQ701086 for 12S rDNA locus and OQ701087-OQ701089 for 18S rDNA locus.
Results and Discussion
Only one male lizard (prevalence = 5.5%) from the Cañas de Michío site was found parasitized with a total of 138 tapeworm larvae, morphologically compatible with tetrathyridia of Mesocestoides (Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018; McAllister et al. Reference McAllister, Tkach and Conn2018). All tetrathyridia were free throughout the coelomic cavity; none was found encysted in the parenchyma or adhered to the serosa of any organ. Three different morphotypes were detected (I, II, and III), all with a well-developed excretory pore and deep invagination canal. Morphotype I had an anterior end wider and more prominent in comparison to its hindbody, with few calcareous corpuscles, mostly on the anterior end. Morphotype II was slender with a smaller anterior end in comparison to its hindbody. Calcareous corpuscles were numerous and concentrated mostly in the anterior end and anterior half of the hindbody. Morphotype III was wider and spherical, with few or no calcareous corpuscles observed (Supplementary Figure 2). Measurements are detailed in Table 1.
Table 1. Measurements of the three morphotypes of tetrathyridia isolated in the braided tree iguana (Liolaemus platei). Mean and standard deviation, with range between parentheses.

* Diameter.
** Measurements of the three morphotypes.
Our 18S sequences showed a 98.96% identity (1145/1157, 100% query cover, 0 gaps, 0 E-value) with Mesocestoides melesi (MN512707) isolated in a bank vole (Myodes glareolus) from Poland, while for 12S locus, our taxon showed 93.22% identity (330/354, 100% query cover, 5 gaps, 1E-141) with Mesocestoides sp. (MG214761) isolated in a Northern bobwhite (Colinus virginianus) from the USA. According to the topology of 18S tree, our sequences were included into the Mesocestoides clade with high nodal support (98% for ML and 0.99 for BI), supporting the morphological diagnosis (Supplementary Figure S3). Conversely, despite the morphological differences abovementioned, the phylogeny of both loci suggests that all three morphotypes are conspecifics (Figures 2, S2), which was also confirmed through the genetic pairwise distances of 12S with 0% of intraspecific differences (Supplementary Table S2). Our sequences formed a monophyletic clade for 12S, with robust nodal support (98% for ML and 1 for BI); meanwhile, for 18S there was mild support for ML (81%) and robust for BI (0.72). The genetic pairwise comparison for 12S showed that our sequences differed by 2.4 to 15.9% of nucleotide positions from other Mesocestoides taxa (Supplementary Table S2).
According to the topology of 12S locus (Figure 1), our sequences were closely related to taxa of Mesocestoides clade C (Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005) (100% for ML, 1 for BI), which includes taxa mostly from the USA, followed by Kazakhstan and Russia. Although there are no available sequences of clade C for 18S locus (see Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005), our sequences showed as a sister taxon (97% for ML, 0.88 for BI) to a clade composed by unidentified Mesocestoides taxa parasitizing domestic dogs, rodents, and shrews from the USA and Russia.
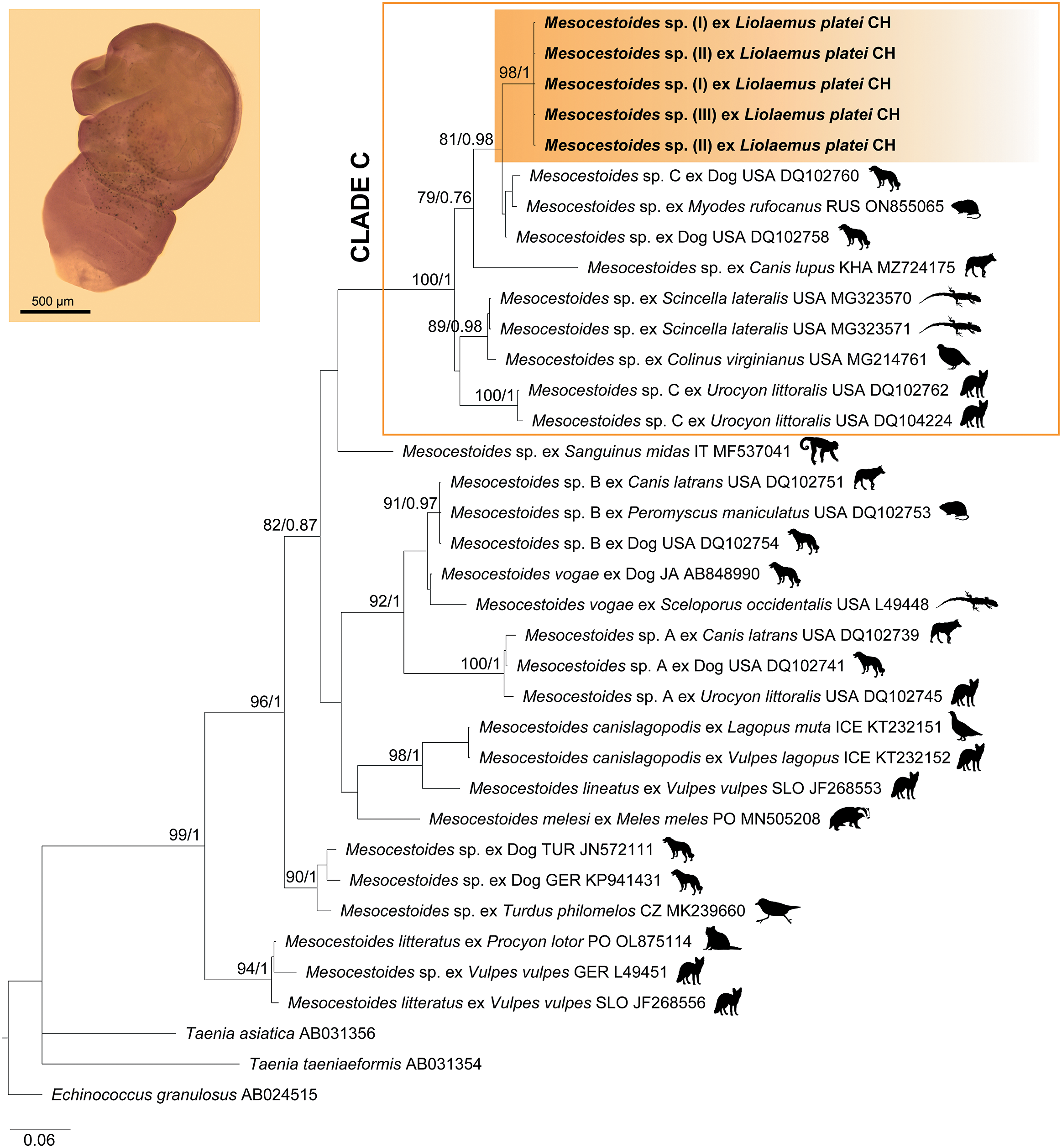
Figure 1. Consensus tree of maximum likelihood (ML) and Bayesian inference (BI) phylogenies inferred for 12S rRNA gene, using an alignment of 315 bp. Calculated substitution models for ML and BI were TPM3u+F+G4, and M85, M15, M90, M50, and M177, respectively. The best models were chosen using the Bayesian Information Criterion (BIC). Bootstrap values and Bayesian posterior probabilities are indicated above each branch. The sequences from the present study are highlighted within an orange box. Abbreviations: CZ, Czech Republic; GER, Germany; ICE, Iceland; IT, Italy; PO, Poland; JA, Japan; KA, Kazakhstan; RUS, Russia; SLO, Slovakia; TUR, Turkey.
The discrepancy between the BLASTn results and the phylogenetic inferences for 18S rRNA could be explained because BLASTn only conducts pairwise sequence comparisons based on local alignment, whereas a phylogenetic approach tries to establish evolutionary relationships using optimal global alignment of all sequences represented, specialized algorithms, and robust statistical methods. Therefore, while BLASTn can be a useful exploratory tool, it should not be considered a substitute for phylogenetic analyses when the goal is to understand the evolutionary relationships among different taxa (Mata et al. Reference Mata, Castañeda, García, Honey, Mendoza and Cervantes2017; Hall Reference Hall2018). On the other hand, the high value of similarity for 18S in the BLASTn result is because this locus is highly conserved in comparison to 12S, which is the reason the former is only informative at the highest level (Crosbie et al. Reference Crosbie, Nadler, Platzer, Kerner, Mariaux and Boyce2000; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018).
The lack of reliable morphological traits for the specific identification of Mesocestoides tetrathyridia has been previously suggested (Skirnisson et al. Reference Skirnisson, Jouet, Ferté and Nielsen2016a, Reference Skirnisson, Sigurðardóttir and Nielsenb; McAllister et al. Reference McAllister, Tkach and Conn2018). As a consequence, the morphological differences of tetrathyridia here reported could be related to different stages of development (Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b), although no larva was determined as a pre-larval stage (see McAllister et al. Reference McAllister, Tkach and Conn2018). García et al. (Reference García, Monachesi and Paz2015) also recorded different morphotypes from a parasitized leopard iguana (Diplolaemus leopardinus) from Argentina; however, the diagnosis was not complemented with a molecular analysis to determine if these were conspecific. Furthermore, the authors described an acephalic morph, which probably corresponded to tetrathyridia with an invaginated scolex (e.g., Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018; present study), because acephalic morph has been found only in domestic dogs (Padget & Boyce Reference Padgett and Boyce2004; Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005).
The prevalence here recorded agrees with the global prevalence of tetrathyridia in lizards, which is near 4% (Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021). The parasitic load recorded is concordant with previous reports, from a few worms to several hundred (Padgett & Boyce Reference Padgett and Boyce2004; Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b). A high number of tetrathyridia could occur during asexual proliferation, as seen in experimental trials with reptiles and micromammals (Padgett & Boyce Reference Padgett and Boyce2004). However, none of the three morphotypes isolated here showed any sign of this, i.e., duplicated scolices or buds (Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018), agreeing with previous records in wild lizards from Europe and North America (McAllister et al. Reference McAllister, Tkach and Conn2018; Berrilli & Simbula Reference Berrilli and Simbula2020). Although asexual proliferation has been recorded for certain populations of Mesocestoides, it seems to be rare in nature (Padgett & Boyce Reference Padgett and Boyce2004; Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018; McAllister et al. Reference McAllister, Tkach and Conn2018; Berrilli & Simbula Reference Berrilli and Simbula2020).
All tetrathyridia were found free in the coelomic cavity, mostly distributed over the digestive tract and liver. No tetrathyridium was found encysted or attached to any organ or to the coelomic wall, corroborating that tissular invasion is not a common strategy in Mesocestoides spp. (Padgett & Boyce Reference Padgett and Boyce2004; present study). Nonetheless, questions arise as to whether this attribute is specific for this taxon or related to the immune response of the host.
Of relevance to the source of infection, the braided tree iguana is catalogued as an insectivorous reptile (Mella Reference Mella2017). Thus, if the life cycle requires an arthropod as the first intermediate host (i.e., a three-host life cycle) (Padgett & Boyce Reference Padgett and Boyce2004), this would explain the infection in lizards. On the other hand, if there is no need for an arthropod (i.e., a two-host life cycle), the lizard could get infected through the ingestion of proglottids, wherein the eggs are protected and concentrated in high numbers inside the paruterine organ (McAllister et al. Reference McAllister, Tkach and Conn2018).
The definitive hosts recorded for clade C are canids, including the domestic dog, Island foxes (Urocyon littoralis), and wolves (Canis lupus) (Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005). Considering that our sequences were closely related to this clade, a similar definitive host would be expected. However, this suggestion must be taken with caution because wild felids have also been recorded with adult tapeworms in South America (Fugassa Reference Fugassa2020).
Parasitological surveys on braided tree iguanas count only ectoparasites (San-Martín-Órdenes et al. Reference San-Martín-Órdenes, Muñoz-Leal, Garín and González-Acuña2019). In consequence, this study represents the first report related to its helminth fauna. Moreover, although there are records of tetrathyridia in other Neotropical reptiles (Bursey et al. Reference Bursey, Goldberg, Telford and Vitt2012; García et al. Reference García, Monachesi and Paz2015; Justo et al. Reference Justo, Fernandes, Cárdenas and Cohen2017), this is the first mention of Liolaemus species acting as the intermediate host of Mesocestoides, expanding the spectrum of intermediate hosts in this region. Furthermore, this is the first attempt to molecularly characterize a tapeworm of genus Mesocestoides from the Neotropics and the third study reporting sequences from a lizard (McAllister et al. Reference McAllister, Tkach and Conn2018; Berrilli & Simbula Reference Berrilli and Simbula2020).
Virtually, all vertebrate preys have the potential to harbour tetrathyridia (Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021). This was supported by the topology for both loci, with the absence of a pattern related to the use of intermediate hosts (Padgett & Boyce Reference Padgett and Boyce2004; Bursey et al. Reference Bursey, Goldberg, Telford and Vitt2012; Skirnisson et al. Reference Skirnisson, Jouet, Ferté and Nielsen2016a, b; Kubečka et al. Reference Kubečka, Traub, Tkach, Shirley, Rollins and Fedynich2018). Some families of lizards that host tetrathyridia elsewhere are also present in Chile, e.g., Gekkonidae, Teiidae, and Scincidae (McAllister et al. Reference McAllister, Tkach and Conn2018; Bursey et al. Reference Bursey, Goldberg, Telford and Vitt2012; Mella Reference Mella2017). Thus, studies focused on the helminthological fauna of these and other families should consider determining the presence of tetrathyridia.
In Chile and Peru there are previous mentions of “M. lineatus” in a domestic dog and Andean fox, respectively (see Fugassa Reference Fugassa2020; Oyarzún-Ruiz et al. Reference Oyarzún-Ruiz, Di Cataldo, Cevidanes, Millán and González-Acuña2020). Considering that this species is restricted to Central Europe (Jesudoss Chelladurai & Brewer Reference Jesudoss Chelladurai and Brewer2021), it is possible that it represents a different taxon. Another conflictive species in the Neotropics is M. variabilis which, besides its natural distribution in the USA, was recorded in an Andean fox from Peru (Angulo-Tisoc et al. Reference Angulo-Tisoc, Curie and Gomez-Puerta2020). According to Padgett et al. (Reference Padgett, Nadler, Munson, Sacks and Boyce2005), this taxon would be synonymous with M. vogae; however, there is no molecular data from the Neotropics to support this statement.
There is the possibility that our specimens represent an undescribed taxon, considering the restricted distribution of the parasitized lizard and the putative wild definitive hosts (Mella Reference Mella2017; Oyarzún-Ruiz et al. Reference Oyarzún-Ruiz, Di Cataldo, Cevidanes, Millán and González-Acuña2020). In addition, future studies in other potential intermediate hosts should prevent overlooking tetrathyridia during necropsy, considering the challenge imposed by its small size and variable morphology, especially with low parasitic loads (e.g., García et al. Reference García, Monachesi and Paz2015; Skirnisson et al. Reference Skirnisson, Sigurðardóttir and Nielsen2016b; McAllister et al. Reference McAllister, Tkach and Conn2018).
This is the first contribution of molecular data for this genus of tapeworm in the Neotropics. Keeping in mind its intricate taxonomic history, additional sequences from this zoogeographical realm could answer questions related to the evolutionary relationships of the genus, particularly those from the Americas (Padgett & Boyce Reference Padgett and Boyce2004; Padgett et al. Reference Padgett, Nadler, Munson, Sacks and Boyce2005; McAllister et al. Reference McAllister, Tkach and Conn2018).
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X23000329.
Acknowledgements
This study is dedicated to the memory of Professor Daniel González-Acuña. The authors appreciate the valuable support given by Adriana Santodomingo, Richard Thomas, Fidel Castro and Lleretny Rodriguez in the laboratory, and Patricio Arroyo in the field.
Financial support
This study was funded by ANID-Vinculación Internacional-FOVI1220125 (S. M.-L. and C. B.-M.), FONDECYT 1221045, ENL01/21 (C. B.-M.), FONDECYT Iniciación 11220177 (S. M.-L.) and FONDECYT Postdoctorado 3230461 (P. O-R).
Competing interest
None.
Ethical standard
Procedures performed in this study were verified and approved by the Bioethics Committee of Universidad de Chile (Form CICUA Nº18202-SCS-UCH). Capture of lizards was authorized by the Servicio Agrícola y Ganadero (SAG; Resolution No. 334/2019).




