Article contents
In situ functionalization of gallium nitride powder with a porphyrin dye
Published online by Cambridge University Press: 27 May 2015
Abstract
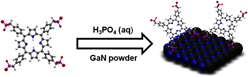
This work focused on the modification of milled GaN powder. Successful attachment of a porphyrin derivative to a GaN powder was performed via in situ functionalization in the presence of phosphoric acid. The GaN powder was imaged using scanning electron microscopy and was found to be heterogeneous in nature, adopting no consistent geometry in the aggregates. The aqueous stability of the porphyrin used was observed in deionized water and a solution of phosphoric acid using ultraviolet–visible spectroscopy. Surface chemistry was characterized with x-ray photoelectron spectroscopy and infrared spectroscopy, which identified evidence of successful functionalization through the presence of characteristic peaks. The interface stability of the covalent bond between GaN and porphyrin was evaluated using fluorescence spectroscopy and demonstrated no leaching of dye in water solutions for 20 days.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 30 , Issue 19: Focus Issue: Nitrides and Oxynitride Materials , 14 October 2015 , pp. 2910 - 2918
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 4
- Cited by


