Article contents
Synthesis of Pt–OMG mesoporous composite via nanocasting and chemical vapor infiltration
Published online by Cambridge University Press: 16 January 2013
Abstract
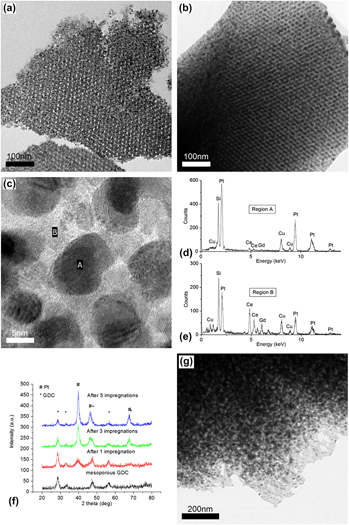
When supported by suitable metal oxides such as ceria, platinum often displays increased catalytic activity and selectivity. A chemical vapor infiltration technique was used to impregnate Pt nanoparticles into an ordered mesoporous gadolinium-doped ceria (OMG), which was templated from KIT-6 silica. High Pt loading, up to 38 vol% of OMG, was achieved. This synthesis method is highly scalable and offers easy control over catalyst–support geometry. A detailed study of the OMG structure was conducted by controlling the synthesis parameters of the KIT-6 silica template. Formation mechanism and thermal stability of the OMG/Pt–OMG composite were also studied. The mesostructure composites were found to sustain until 750 and 650 °C, respectively. The highly structural composite holds the promise of increased activity, selectivity, and stability for applications in heterogeneous catalysis.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 3
- Cited by


