I. INTRODUCTION
Hydrogen evolution reaction (HER) and oxygen reduction reaction (ORR) are of great interest due to their important roles in renewable energy conversion and storage systems. Reference Aricò, Bruce, Scrosati, Tarascon and Schalkwijk1–Reference Zhang, Zhao, Xia and Dai3 High-performance of HER and ORR is limited due to the activity of the catalysts. Reference Zhao, Yin, Du, He, Zhao, Chang, Yin, Zhao, Liu and Tang4–Reference Gong, Du, Xia, Durstock and Dai6 Traditionally, precious metals (e.g., platinum) are efficient for HER and ORR with both activity and stability. Reference Duan, Bozoglian, Mandal, Stewart, Privalov, Llobet and Sun7–Reference Zhang, Hwang, Trout and Peng9 However, their high cost and scarcity have hindered their large-scale application. Reference Dai, Xue, Qu, Choi and Baek10–Reference Liu, Wu, Miao and Yang13 What is more, the design of an ideal electrocatalyst that can drive both HER and ORR with high current density at low overpotential and long-term stability is still a challenge. Reference Mefford, Rong, Abakumov, Hardin, Dai, Kolpak, Johnston and Stevenson14–Reference Cao, Zhao, Yu, Chen, Huang, Yang, Cao, Lu, Zhang, Zhang, Tan and Zhang16 To address these challenging issues, many efforts have been focused on non-precious-metal–based materials such as perovskite, metallic oxide, and their derivatives as bifunctional electrocatalysts, Reference Hiralal, Imaizumi, Unalan, Matsumoto, Minagawa, Rouvala, Tanioka and Amaratunga15–Reference Ma, Cao, Jaroniec and Qiao20 as exploring strategies to develop more efficient and stable catalysts in a cost-effective manner is still a technological task in the development of energy conversion and storage systems.
The development of energy conversion and storage systems take an important part in daily life because of the energy crisis and ecological environment pollution. Metal–air batteries as potential alternative energy sources have been the subject of intense investigation in recent years. Reference Chen, Duan, Bian, Tang, Zheng and Qiao21–Reference Xia, Jiang, Zhao, Hedhili and Alshareef25 Theoretically, Zn–air batteries, as one of most widespread metal–air batteries, have high energy density (1086 W h/kg, including oxygen) and low cost and are environmentally friendly. Reference Xia, Jiang, Zhao, Hedhili and Alshareef25–Reference Yin, Fan, Li, Cheng, Zhou, Xi and Sun30 However, the obtained electrochemical performances are still far from satisfying because the energy efficiency is still limited by the large overpotentials derived from the sluggish oxygen reduction and evolution reaction (ORR/OER) kinetics, the unstable electrode structure even further fueling it. Reference Wang, Guo, Zhou, Chang, Zheng and Li31–Reference Zhang, Ouyang, Xu, Chen, Rawat and Fan33 Therefore, the development of excellent air electrode with high catalytic activity, good conductivity, and favorable mechanical properties is of great importance in enhancing the energy density, powder density, and rechargeable capacity for the Zn–air batteries.
Among various transition metal compound catalysts, the spinel AB2O4 (A, B = metal) possesses high electrochemical activity and superior adsorption and activation of electroactive species, which can be attributed to surface redox active metal centers by electron transition between different valence states of metals in O-sites. Reference Huang, Chen, Ding, Feng, Wang and Liu34–Reference An, Huang, Zhou, Yin, Liu and Xi36 As one of the most intriguing spinel composite oxides, CoFe2O4 is widely used because of many advantages, including low cost, high abundance, low toxicity, and rich redox chemistry. Reference An, Huang, Zhou, Yin, Liu and Xi36–Reference Bediako, Lassalle-Kaiser, Surendranath, Yano, Yachandra and Nocera38 CoFe2O4 also displays a boosting electrocatalytic performance due to the abundant metal ions with different valence states (e.g., Fe2+, Fe3+, Co2+, Co3+). As we all know, various morphologies of CoFe2O4 have been researched in many fields. Reference Bediako, Lassalle-Kaiser, Surendranath, Yano, Yachandra and Nocera38–Reference Nam, Bak, Hu, Yu, Zhou, Wang, Wu, Zhu, Chung and Yang40 However, the performance of CoFe2O4 is still limited by morphology and active crystal face exposed. Recently, many researches have been devoted to fabricating the more active and uniform nanocrystals. Furthermore, many studies revealed that crystal face such as (100), (110), and (111) with low surface energy have been well documented in literature.
Herein, we report the uniform CoFe2O4 nanoparticles with abundant oxygen vacancies and the specific active surface exposed as active and stable bifunctional electrocatalysts for boosting HER and ORR through a simple hydrothermal method. The high electrocatalytic activities of the CoFe2O4 nanoparticles for HER and ORR are caused by their uniform nanoparticle morphology, the active crystal face (111) and (110) exposed, and abundant oxygen vacancies. As expected, they show excellent performance for both HER in both acidic and basic media and ORR in 0.1 M KOH. In detail, they show superior activity for HER in acid media with an onset potential of 20 mV, overpotential of 45 mV (at j = 10 mA/cm2), remarkable long-term stability more than 100 h with different current densities, and rapid kinetic reaction with the lower Tafel slope of 35 mV/dec. What is more, the CoFe2O4 nanoparticles also displayed a better performance for HER in basic media with an overpotential of 65 mV (at j = 10 mA/cm2). The bifunctional electrocatalytic capacity of CoFe2O4 nanoparticles was confirmed by good performance for ORR. Both the onset potential (0.84 V) and half-wave potential (0.67 V) of the CoFe2O4 nanoparticles for ORR are closer to that of Pt/C (20%), and more importantly, the ORR is catalyzed by the CoFe2O4 nanoparticles via four electrons processes. Based on the bifunctional electrocatalytic performance, the primary home-made Zn–air batteries were fabricated, using CoFe2O4 nanoparticles as the air–cathode. More interestingly, the two-series-connected batteries fabricated by CoFe2O4 nanoparticles can support a light-emitting diode (LED) to work for more than 48 h.
II. EXPERIMENTAL SECTION
A. Materials
Fe(NO3)3·9H2O (98.0%), Co(NO3)2·6H2O (99.0%), NaCH3COO (99.0%), H2SO4 (99.0%), KOH (90.0%), and polyethylene glycol 200 (PEG-200) (90.0%) were purchased from Aladdin Nafion (5%; Los Angeles, California) and carbon black was purchased from Sigma-Aldrich (St. Louis, Missouri) and Pt/C catalyst (20 wt% Pt on carbon black) from Premetek Co. (Wilmington, Delaware); all of these materials are of analytical grade and without further purification. The deionized (DI) water for solution preparation was obtained from a Millipore Autopure system (18.2 MΩ, Millipore Ltd., Billerica, Massachusetts). The 0.5 M H2SO4, and 0.1 and 1.0 M KOH were used as the electrolyte in the electrochemical experiment.
B. Fabrication of CoFe2O4 nanoparticles
The CoFe2O4 nanoparticles were prepared by a facile hydrothermal synthesis method. Generally speaking, Fe(NO3)2·9H2O (10 mmol, 4.0401 g), Co(NO3)2·6H2O (5 mmol, 1.4553 g), and NaCH3COO (30 mmol 2.46 g) were dissolved in 40 mL of polyethylene glycol 200 (PEG-200). The clear solution of these materials was obtained after stirring for about 30 min. Then the solution was transferred into the Teflon-lined stainless steel (80 mL) in an autoclave. After autoclave treatment at 200 °C for 16 h, the autoclave was allowed to cool down to room temperature. The resulting precipitates were filtered and washed thoroughly with DI water and ethanol more than three times and then dried in air at 80 °C for 12 h. These precipitates were calcined in air at 400 °C for 2 h to obtain CoFe2O4 nanoparticles.
C. Structure characterization
X-ray diffraction (XRD) experiments were conducted by the X’Pert Pro X-ray diffractometer with Cu Kα radiation (λ = 0.1542 nm) under a voltage of 40 kV from 20° to 90°. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) specimens were prepared by depositing a drop of the CoFe2O4 nanoparticles dispersion in H2O on copper grids coated with amorphous carbon. TEM and HRTEM observations were performed under an acceleration voltage of 200 kV with a JEOL JEM 2100 TEM (JEOL, Ltd., Tokyo, Japan). X-ray photoelectron spectroscopy (XPS) analyses were made with a VG ESCALAB 220I-XL device (VG Instruments, Middlewich, United Kingdom) and corrected with C 1s line at 284.6 eV.
D. The electrocatalytic performance test
All the electrochemical measurements were carried out in a typical three-electrode system consisting of a glass carbon working electrode, a Pt auxiliary electrode, and an Ag/AgCl reference electrode (3 M KCl) connected to a CHI 760 E electrochemical workstation (CHI Instruments, Shanghai Chenhua Instrument Corp., Shanghai, China) at a scan rate of 2 mV/s in the electrolyte solution. The potentials were referenced to the reversible hydrogen electrode (RHE) through RHE calibration [E(RHE) = E(Ag/AgCl) + 0.0951 pH + 0.197]. Also, a resistance test was made, and the iR compensation was applied using the CHI software. In this work, all electrochemical experiments were conducted at 20 ± 0.2 °C. The working electrodes were prepared in the same way for both HER and ORR. First, the catalysts (3 mg) were dispersed in a N,N-dimethylformamide (DMF) solution (1470 μL) with 30 μL of Nafion solution (Aldrich, 5 wt%) by sonication for at least 40 min to form a homogeneous ink. Then, 7 μL of the dispersion was loaded onto a glassy carbon electrode with a diameter of 3 mm (loading 0.2 mg/cm2), and 12.6 μL of the dispersion was loaded onto the rotating disk electrode (RDE) or rotating ring-disk electrode (RRDE) with a diameter of 4 mm (loading 0.2 mg/cm2). The electrochemical measurements for ORR were conducted on RRDE at 1600 rpm in oxygen-saturated 0.1 M KOH solution. The RDE was measured at rotating rates varying from 400 to 2400 rpm at the scan rate of 2 mV/s. The relationship between the measured currents (j) with various rotating speeds (ω) under fixed potentials can be expressed on the basis of the Koutechky–Levich (K–L) equation Reference Zhang, Qu, Shi, Liu, Chen and Dai41 :
where j k is the kinetic current and ω is the electrode rotating rate. B is determined from the slope of the K–L plots based on the Levich equation below:
where n represents the transferred electron number per oxygen molecules.
![]() ${D_{{{\rm{O}}_2}}}$
is the diffusion coefficient of O2 in 0.1 M KOH (
${D_{{{\rm{O}}_2}}}$
is the diffusion coefficient of O2 in 0.1 M KOH (
![]() ${D_{{{\rm{O}}_2}}}$
= 1.9 × 10−5 cm2/s). v is the kinetic viscosity (v = 0.01 cm2/s).
${D_{{{\rm{O}}_2}}}$
= 1.9 × 10−5 cm2/s). v is the kinetic viscosity (v = 0.01 cm2/s).
![]() ${C_{{{\rm{O}}_2}}}$
is the bulk concentration of O2 (
${C_{{{\rm{O}}_2}}}$
is the bulk concentration of O2 (
![]() ${C_{{{\rm{O}}_2}}}$
= 1.2 × 10−6 mol/cm3). The constant 0.2 is adopted when the rotation speed is expressed in rpm.
${C_{{{\rm{O}}_2}}}$
= 1.2 × 10−6 mol/cm3). The constant 0.2 is adopted when the rotation speed is expressed in rpm.
For the RRDE measurements, catalyst inks and electrodes were prepared by the same method as for RDE. The disk electrode was scanned at a rate of 5 mV/s, and the ring potential was constant at 1.3 V versus RHE. The %
![]() ${\rm{OH}}_2^ -$
and transfer number (n) were determined by the following equations
Reference Zhang, Qu, Shi, Liu, Chen and Dai41
:
${\rm{OH}}_2^ -$
and transfer number (n) were determined by the following equations
Reference Zhang, Qu, Shi, Liu, Chen and Dai41
:
where I d is disk current, I r is ring current, and N is current collection efficiency of the Pt ring. N was determined to be 0.40.
E. Battery test
The Zn–air battery was assembled by the zinc plate anode and air–cathode. The air–cathode was prepared by dropping the CoFe2O4 nanoparticle homogeneous catalyst ink on the carbon fiber paper (CFP) and drying in air. First, the catalysts (9 mg) were dispersed in a DMF solution (4410 μL) with 120 μL of Nafion solution (Aldrich, 5 wt%) by sonication for at least 40 min to form a homogeneous ink. Then, 4 mL of the dispersion was loaded onto the CFP with 1 × 2 cm2 (loading 2 mg/cm2). Last, the catalyst could be dried in air. The 6.0 M KOH was used as the electrolyte. All the Zn–air batteries were tested under ambient atmosphere. The polarization curve measurements were performed by linear scan voltammogram (LSV) (2 mV/s) at 25 °C with CHI 760 E electrochemical working station (CH Instrument). Reference Liu, Cheng, Li, Ma and Su5,Reference Fominykh, Chernev, Zaharieva, Sicklinger, Stefanic, Döblinger, Müller, Pokharel, Böcklein, Scheu, Bein and Fattakhova-Rohlfing11
III. RESULTS AND DISCUSSION
A. Synthesis and characterization of CoFe2O4 nanoparticles
A strategy was developed to synthesize CoFe2O4 nanoparticles by the hydrothermal method at 200 °C for 16 h (Experimental Section).
Reference Jin, Wang, Su, Wei, Pang and Wang42
Figure 1(a) shows the XRD pattern of the CoFe2O4 nanoparticles. The diffraction peaks of CoFe2O4 nanoparticles belong to the space groups of
![]() $F\bar{4}3m$
(JCPDS Card No. 1-1121; a = b = c = 8.39 Å) with cubic structure. In detail, as shown in Fig. 1(a), the main crystal face of CoFe2O4 could be obviously seen, such as the (220) crystal face at the angle of 30.39°, the (311) crystal face at the angle of 35.78°, the (400) crystal face at the angle of 43.53°, the (511) crystal face at the angle of 57.18°, and the (440) crystal face at the angle of 62.74°, respectively. The morphology and structure of the CoFe2O4 nanoparticles were confirmed by TEM. As shown in Fig. 1(b), the TEM results reveal that the CoFe2O4 nanoparticles have uniform nanoparticles morphology with 5–10 nm. Furthermore, the HRTEM was used to study the exposed crystal face of the CoFe2O4 nanoparticles. As shown in Fig. 1(c), two clearly identified lattice fringe spaces of 2.97 and 4.86 Å correspond to the (220) plane and (111) plane exposed for the CoFe2O4 nanoparticles. Based on the above analysis, the CoFe2O4 nanoparticles with uniform small nanoparticles morphology and active crystal face exposed will have an excellent performance for electrocatalysis.
$F\bar{4}3m$
(JCPDS Card No. 1-1121; a = b = c = 8.39 Å) with cubic structure. In detail, as shown in Fig. 1(a), the main crystal face of CoFe2O4 could be obviously seen, such as the (220) crystal face at the angle of 30.39°, the (311) crystal face at the angle of 35.78°, the (400) crystal face at the angle of 43.53°, the (511) crystal face at the angle of 57.18°, and the (440) crystal face at the angle of 62.74°, respectively. The morphology and structure of the CoFe2O4 nanoparticles were confirmed by TEM. As shown in Fig. 1(b), the TEM results reveal that the CoFe2O4 nanoparticles have uniform nanoparticles morphology with 5–10 nm. Furthermore, the HRTEM was used to study the exposed crystal face of the CoFe2O4 nanoparticles. As shown in Fig. 1(c), two clearly identified lattice fringe spaces of 2.97 and 4.86 Å correspond to the (220) plane and (111) plane exposed for the CoFe2O4 nanoparticles. Based on the above analysis, the CoFe2O4 nanoparticles with uniform small nanoparticles morphology and active crystal face exposed will have an excellent performance for electrocatalysis.
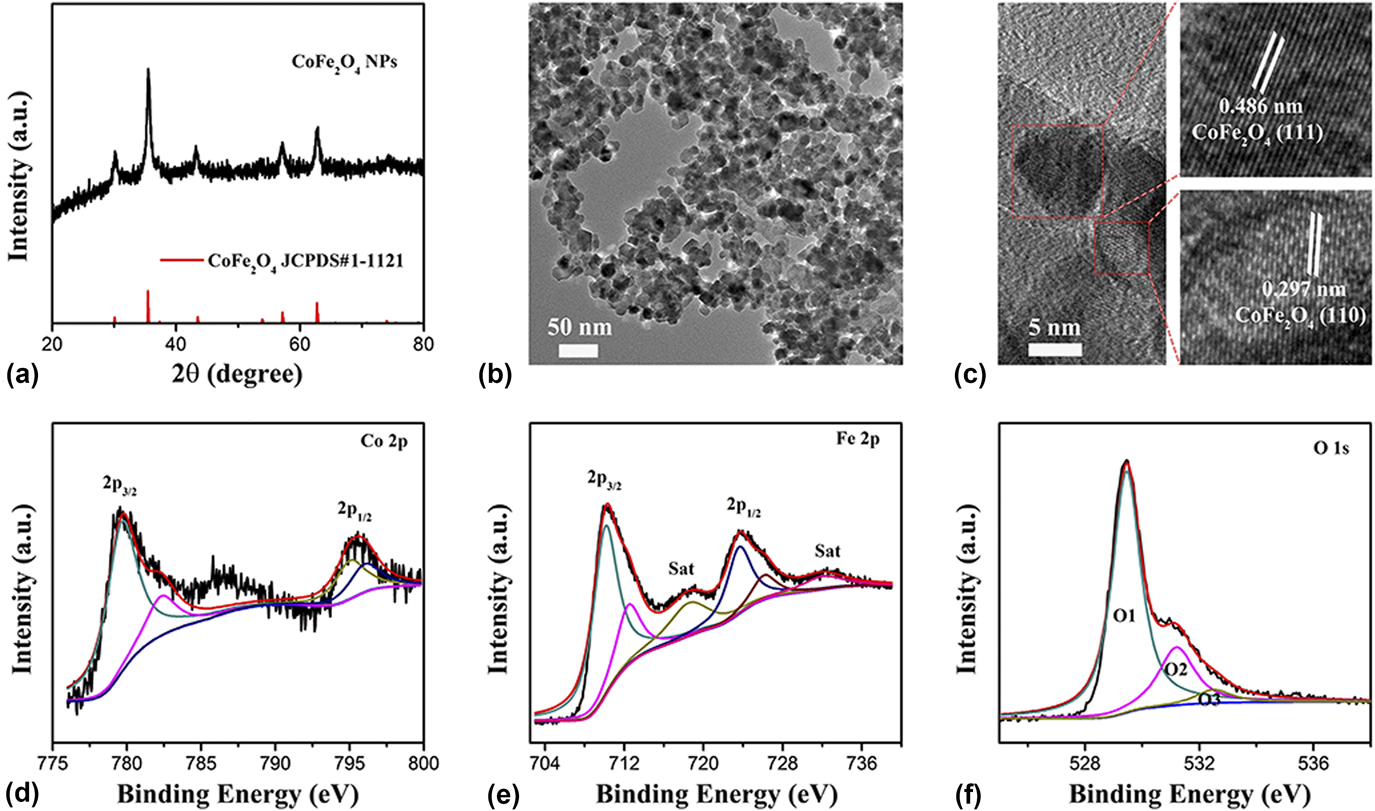
FIG. 1. (a) XRD patterns, (b) TEM, and (c) HRTEM images of CoFe2O4 nanoparticles. XPS spectra of (d) Co 2p, (e) Fe 2p, and (f) O 1s for the CoFe2O4 nanoparticles.
XPS measurements were also used to investigate the surface properties of the CoFe2O4 nanoparticles. In Fig. 1(d), the Co 2p spectrum of CoFe2O4 nanoparticles consists of two spin–orbit doublets characteristic of Co2+ and Co3+ and shakeup satellites (identified as “Sat.”), which can be observed at 795.48 eV for Co 2p 1/2 peaks and 779.78 eV Co 2p 3/2 with spin–orbit splitting of 15.7 eV. Additionally, there is a shake-up satellite peak at 786.55 eV. Reference Wang, Zhuo, Liang, Han and Zhang43,Reference Zhang, Ouyang, Xu, Jia, Chen, Rawat and Fan44 The energy gap of Co is about 8.93 eV which can be calculated by the difference value of binding energy between the Co 2p main peak and the satellite peak, meaning the Co cation holds a valence of Co3+. Reference Zhang, Ouyang, Xu, Chen, Rawat and Fan33 Similarly, the Fe 2p spectrum of CoFe2O4 nanoparticles [Fig. 1(e)] also consist of two spin–orbit peaks and corresponding Sat. of Fe2+ at high field and of Fe3+ at low field. Two main peaks located at 723.73 and 710.30 eV are Fe 2p 1/2 and Fe 2p 3/2 with the corresponding Sat. at 732.08 eV and 718.65 eV, Reference Sharma, Sharma, Rao and Chowdari45,Reference Ma, Dai, Jaroniec and Qiao46 respectively. Figure 1(f) shows the O1s spectrum of CoFe2O4 nanoparticles. In detail, it shows three oxygen contributions, denoted as O1 (529.46 eV) for oxygen atoms bound to metals (O-Fe/Co), O2 (531.22 eV) attributed to the oxygen vacancy (Ov), and O3 (532.52 eV) connected with surface-adsorbed water molecules (O–H2O). Reference McCrory, Jung, Peters and Jaramillo47 Extensive studies reveal that highly active nanostructures with abundant oxygen vacancies play an important role in enhancing the performance of the electrocatalytic reaction and renewable-energy devices due to their beneficial adsorption energy of the intermediate reaction, favorable desorption energy of the products, and efficient proton electron conduction. The CoFe2O4 nanoparticles with uniform small nanoparticles morphology, exposed active crystal face, and abundant oxygen vacancies will have an excellent performance for electrocatalysis and renewable-energy devices.
B. Hydrogen evolution reaction catalytic performance
The HER activity of the CoFe2O4 nanoparticles and commercial Pt/C (20 wt% Pt on carbon black from Premetek. Co., mass loading of 0.2 mg/cm2) was confirmed by LSV both in acidic and basic media with the scan rate of 2 mV/s (Experimental Section). Reference Chen, Xu, Fang, Tong, Wu, Lu, Peng, Ding, Wu and Xie48 Figure 2(a) shows that the CoFe2O4 nanoparticles exhibit the onset potential of 20 mV (versus RHE) slightly higher than that of commercial Pt/C (20%) (0 V versus RHE), and the overpotential of 45 mV (at j = 10 mA/cm2) a little more than that of commercial Pt/C (20%) (14 mV versus RHE), indicating that the catalytic behavior of the CoFe2O4 nanoparticles is similar to that of the commercial Pt/C catalyst. Reference Gao, Cao, Gao, Xu, Zheng, Jiang and Yu49 Figure 2(b) displays the Tafel plots (log j ∼ η) of HER. The mechanism typically involves three major reactions for hydrogen evolution in acid solutions on metal electrode surfaces. Reference Yin, Zhou, An, Huang, Shao, Wang, Liu and Xi35
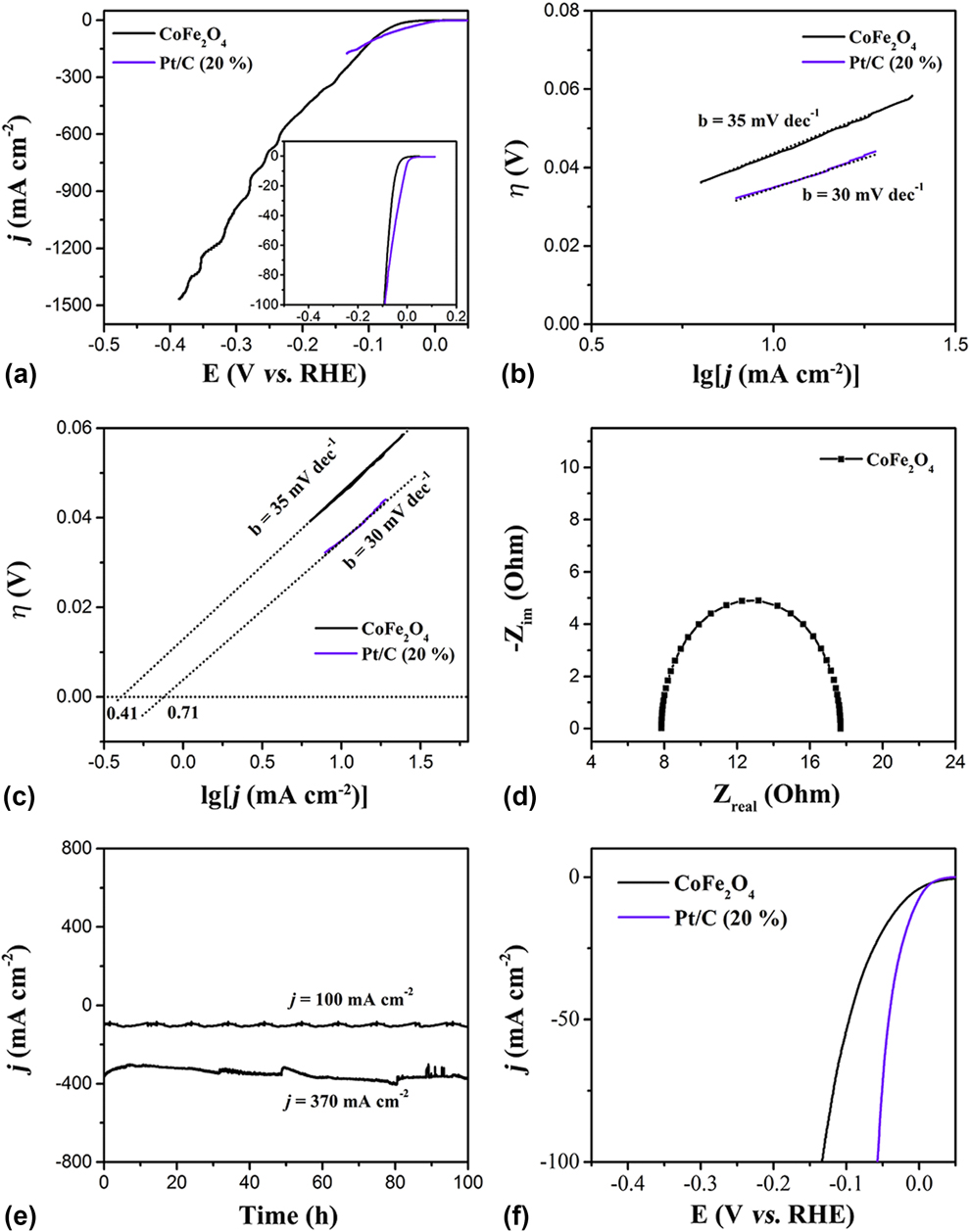
FIG. 2. (a) LSV curves of CoFe2O4 nanoparticles and Pt/C (20%) for hydrogen evolution in 0.5 M H2SO4, (b) the corresponding Tafel curves, and (c) the exchange current densities. (d) The Nyquist plot of the CoFe2O4 nanoparticles for HER. (e) HER stability tests of the CoFe2O4 nanoparticles at a different potential. (f) LSV curves of the samples for hydrogen evolution in 1 M KOH.
In these reaction processes, the metal-bound electrons are named e-catalyst, and a hydrogen atom or molecule adsorbed on to the surface of the metal atom catalysts is denoted as a H-catalyst or H2-catalyst, respectively. These reactions are dependent on the inherent (electro)chemical and electronic properties of the catalyst surface. Generally, two main pathways are observed in HER, which are Volmer–Tafel reaction [Eqs. (5) and (6)] and Volmer–Heyrovsky reaction [Eqs. (5) and (7)]. In this work, the HER on the Pt/C (20%) with a Tafel slope of 30 mV/dec follows the known Tafel mechanism (Hads + Hads → H2↑). Reference Gao, Sheng, Zhuang, Fang, Gu, Jiang and Yan50 Similarly, the CoFe2O4 nanoparticles have a very close Tafel slope of 35 mV/dec to that of Pt/C (20%), suggesting that the HER on this catalyst is dominated by the Heyrovsky mechanism (H2O + MHad ⇌ M + H2 + OH−) with electrochemical desorption step as the rate-determining step. Reference Gong, Li, Wang, Liang, Wu, Zhou, Wang, Regier, Wei and Dai51 Noticeably, the CoFe2O4 nanoparticles show an excellent performance for HER in acid media. In addition to the exchange current densities (j 0) of the CoFe2O4 nanoparticles and Pt/C (20%), the most inherent measure of HER activity was further calculated by extrapolating the Tafel plots to assess the quality of the electrocatalysts. As seen in Fig. 2(c), the j 0 value of 0.41 mA/cm2 for the CoFe2O4 nanoparticles is slightly less than the value of 0.71 mA/cm2 for Pt/C (20%). Meanwhile, electrochemical impedance spectroscopy (EIS) was tested in 0.5 M H2SO4 solution to provide further insight into the electrode kinetics of the CoFe2O4 nanoparticles during the HER process. The Nyquist plot for the CoFe2O4 nanoparticles was measured in the 0.5 M H2SO4 solution from 0.01 to 100,000 Hz at 0.1 V. Circuit model fitting analysis of the EIS suggests that both electrodes can be modeled using a modified equivalent circuit consisting of a series resistance (R s), a constant phase element, and a charge transfer resistance (R ct). Figure 2(d) reveals that the R ct of CoFe2O4 nanoparticles is only ∼10 Ω, which is a very small observed R ct value, suggesting that the CoFe2O4 nanoparticles catalyst possesses a rapid electron transport during the HER process. The high j 0 value and the small R ct signal suggest that the CoFe2O4 nanoparticles act as a promoter, facilitating the charge transfer on the surface to achieve efficient conversion of H+ into H2 bubbles.
Stability as another important factor in all electrocatalytic reactions was also measured. As shown in Fig. 2(c), because of the uniform nanoparticles morphology, the exposed active crystal face of (111) and (220), and the abundant oxygen vacancies, the CoFe2O4 nanoparticles showed a superior stability for HER no less than 100 h at different current densities of 100 and 370 mA/cm. Many research have studied the HER performance of various catalysts in acid media, but the overall water splitting usually takes place in basic media. Therefore, the HER performance of the CoFe2O4 nanoparticles in basic media was also confirmed. As shown in Fig. 2(c), the CoFe2O4 nanoparticles show a remarkable activity for HER. Furthermore, the performance of CoFe2O4 nanoparticles for HER in basic media was also confirmed. The CoFe2O4 nanoparticles show better performance for HER in 1.0 M KOH solution with an overpotential of 65 mV at j = 10 mA/cm closer to that of Pt/C (20%). Based on the above analysis, the CoFe2O4 nanoparticles show an excellent performance for HER in both acidic and basic media.
The superior HER catalytic performance of the CoFe2O4 nanoparticle electrodes can be attributed to the uniform small nanoparticle structure, the exposed active crystal face of (111) and (220), and the abundant oxygen vacancies. First, the uniform small nanoparticle structure of materials is very important in electrocatalysis. Second, the exposed active crystal face of (111) and (220) for the CoFe2O4 nanoparticles endows low surface energy and better adsorption energy during the electrocatalytic reaction. Third, benefiting from the abundant oxygen vacancies, the reaction kinetics of HER can be significantly promoted. Also, these excellently provide a straightway path for electron transport and enhance structural stability. Therefore, the collaborative advantages enable CoFe2O4 nanoparticles to achieve excellent HER catalytic activity.
C. Oxygen reduction reaction catalytic performance
The electrocatalytic performance of the CoFe2O4 nanoparticles for the ORR was further measured to confirm the bifunctional electrocatalytic capacity and potential application in renewable energy technologies. The as-synthesized CoFe2O4 nanoparticles were coated on a RDE to investigate the ORR performance in an aqueous solution of 0.1 M KOH (Experimental Section). Meanwhile, the ORR electrocatalytic activity of commercial Pt/C (20%) was also tested as a contrast. Reference Bao, Zhang, Fan, Zhang, Zhou, Yang, Hu, Wang, Pan and Xie52 Figure 3(a) shows the LSV curves measured at a rotating speed of 1600 rpm; these results indicate that the ORR catalytic activity of CoFe2O4 nanoparticles is comparable to the Pt/C catalyst with only slightly more negative onset potential [the CoFe2O4 nanoparticles: 0.84 V, Pt/C (20%): 0.95 V] and half-wave potential [the CoFe2O4 nanoparticles: 0.67 V, Pt/C (20%): 0.78 V]. The Tafel slopes of the CoFe2O4 nanoparticles and Pt/C (20%) were also tested to study the kinetics for the ORR. As shown in Fig. 3(b), the Tafel slopes of catalysts for ORR with the CoFe2O4 nanoparticles display the Tafel slope of 101 mV/dec higher than that of Pt/C (20%) (60 mV/dec), suggesting a lower reaction kinetics of the CoFe2O4 nanoparticles for ORR.
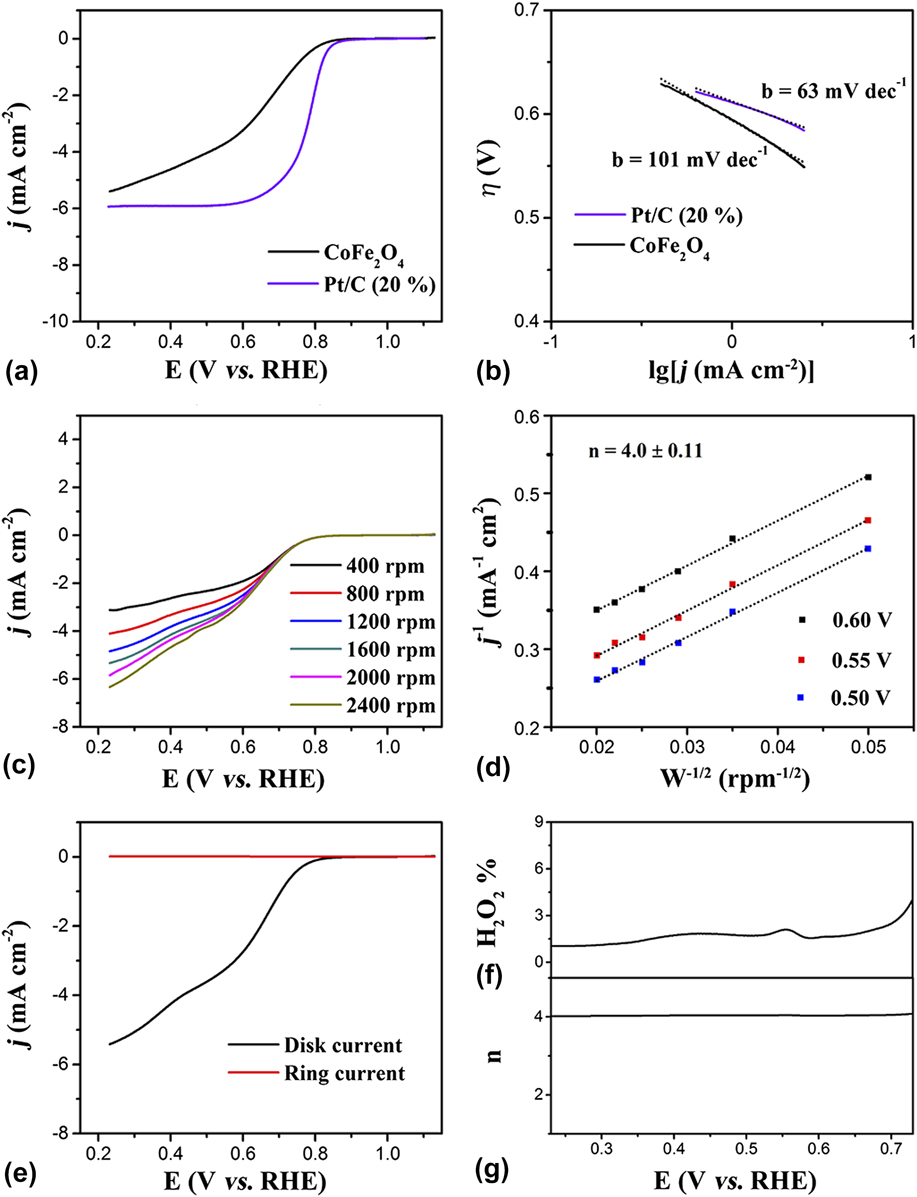
FIG. 3. (a) LSV curves of CoFe2O4 nanoparticles and Pt/C (20%) catalyst in O2-saturated 0.1 M KOH solution and (b) corresponding Tafel curves. (c) LSV curves of the CoFe2O4 nanoparticles in O2-saturated 0.1 M KOH with different rotating speeds. (d) The K–L plots for CoFe2O4 nanoparticles at a different potential. (e) RRDE measurements for the CoFe2O4 nanoparticles electrode in O2-saturated 0.1 M KOH. (f) Percentage of peroxide and (g) the number of electron transfer in the total oxygen reduction progress.
More importantly, the ORR performance of the CoFe2O4 nanoparticles was tested at different rotating speeds from 400 to 2400 rpm to study the relationship between the measured current densities (j) and various rotating speeds under fixed potentials. As shown in Fig. 3(c), the onset potential of the CoFe2O4 nanoparticles for ORR was the same as 0.84 V at different rotating speeds, but the half-wave potential has a slight difference at different rotating speeds such as 0.65 V at the rotating speeds of 400 rpm, 0.66 V at the rotating speeds of 800 rpm, 0.67 V at the rotating speeds of 1200 rpm, 0.68 V at the rotating speeds of 2000 rpm, and 0.69 V at the rotating speeds of 2400 rpm. However, the limited current densities have an obvious difference for every rotating speed, for example, 3.11 mA/cm2 at rotating speeds of 400 rpm, 4.11 mA/cm2 at rotating speeds of 800 rpm, 4.84 mA/cm2 at rotating speeds of 1200 rpm, 5.34 mA/cm2 at rotating speeds of 1600 rpm, 5.85 mA/cm2 at rotating speeds of 2000 rpm, and 6.34 mA/cm2 at rotating speeds of 2400 rpm, suggesting the better active performance at a higher rotating speed [Fig. 3(c)].
Based on the analysis about the relationship of current densities and various rotating speeds, the K–L plots of CoFe2O4 nanoparticles for ORR was calculated [Eqs. (1) and (2) in the Experimental Section]. Reference Lei, Sun, Liu, Gao, Liang, Pan and Xie53–Reference Meng, Zhong, Bao, Yan and Zhang55 As shown in Fig. 3(d), the K–L equation at three different potentials was calculated. In detail, at 0.5 V, the calculated kinetic current densities were 0.429 mA/cm2 at rotating speeds of 400 rpm, 0.348 mA/cm2 at rotating speeds of 800 rpm, 0.308 mA/cm2 at rotating speeds of 1200 rpm, 0.283 mA/cm2 at rotating speeds of 1600 rpm, 0.273 mA/cm2 at rotating speeds of 2000 rpm, and 0.261 mA/cm2 at rotating speeds of 2400 rpm. When the potential was set at 0.55 V, the calculated kinetic current densities were slightly enhanced, such as 0.465 mA/cm2 at rotating speeds of 400 rpm, 0.383 mA/cm2 at rotating speeds of 800 rpm, 0.340 mA/cm2 at rotating speeds of 1200 rpm, 0.315 mA/cm2 at rotating speeds of 1600 rpm, 0.308 mA/cm2 at rotating speeds of 2000 rpm, and 0.292 mA/cm2 at rotating speeds of 2400 rpm. Furthermore, the larger kinetic current densities can be obtained at 0.6 V, such as 0.521 mA/cm2 at rotating speeds of 400 rpm, 0.442 mA/cm2 at rotating speeds of 800 rpm, 0.400 mA/cm2 at rotating speeds of 1200 rpm, 0.377 mA/cm2 at rotating speeds of 1600 rpm, 0.360 mA/cm2 at rotating speeds of 2000 rpm, and 0.351 mA/cm2 at rotating speeds of 2400 rpm, respectively. More importantly, according to the K–L equation yield the electron transferred number (n) of the CoFe2O4 nanoparticles for ORR was calculated to approximately 4 at different potential. Based on the above analysis, the CoFe2O4 nanoparticles showed an outstanding performance for ORR.
Furthermore, the RRDE measurements were also used to study the ORR performance for CoFe2O4 nanoparticles electrode. First, just the same with RDE measurement, the same uniform mass of CoFe2O4 nanoparticle catalyst ink was coated on the RRDE. Then, ORR took place on disk electrode and peroxide generation created on the Pt ring electrode which is set at a constant potential of 1.3 V versus RHE (Experimental Section). Fortunately, under the accurate measurement experiment, the CoFe2O4 nanoparticles exhibit good performance for ORR with a large disk current density and negligible current information of peroxide generation [Fig. 3(e)]. In addition, the percentage of peroxide generation during ORR progress was calculated from the RRDE data [Eq. (3) in the Experimental Section] and is lower than 10% from the potential from 0.23 to 0.73 V, indicating that during the ORR progress, the peroxide generation could be negligible [Fig. 3(f)]. Similarly, as shown in Fig. 3(g), the electron transferred number during the ORR progress is approximately 4, according to the RRDE data calculated results [Eq. (4) in the Experimental Section]. Thus, both the RDE and RRDE measurements suggest an efficient oxygen reduction directly into water via a four-electron pathway. As is well known, excellent catalytic properties are determined by a highly active structure. The excellent ORR catalytic performance of the CoFe2O4 nanoparticle electrodes can be attributed to the uniform small nanoparticle structure, the exposed active crystal face of (111) and (220), and the abundant oxygen vacancies. Except for the uniform small nanoparticle structure, the abundant oxygen vacancies have an important role during the ORR progression for the CoFe2O4 nanoparticles. First, the oxygen vacancy is conducive to the adsorption of hydroxyl radicals in the basic media and reducing the adsorption energy at the same time, suggesting the active and fast electrocatalytic performance for ORR. Additionally, the exposed active crystal face of (111) and (220) for the CoFe2O4 nanoparticles also have a nonnegligible effect for ORR. Therefore, these collaborative advantages enable the CoFe2O4 nanoparticles to achieve excellent HER and ORR catalytic activity and stability. Based on the above experiment and analysis, the uniform CoFe2O4 nanoparticles with abundant oxygen vacancies and exposed active crystal face of (111) and (220) show excellent electrocatalytic performance for both HER and ORR and would have a better performance in renewable energy conversion and storage systems.
D. Battery performance
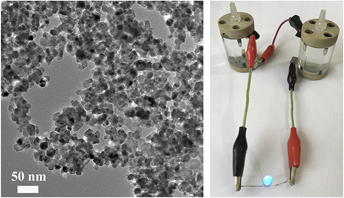
Considering the excellent bifunctional catalytic activity of the CoFe2O4 nanoparticles for both HER and ORR, a home-made primary Zn–air battery was assembled to further identify its performance under practical battery operation conditions. In detail, the battery uses the zinc plate as the anode, the CoFe2O4 nanoparticles coated on the CFP as the air–cathode, and 6.0 M KOH as the electrolyte (Experimental Section). Reference Nam, Park, Choi, Oh, Park, Kim, Park, Cho and Lee54–Reference Park, Park, Nam, Lee and Cho56 Figure 4(a) shows the voltages and powder densities with current densities. When the current density is zero, the Zn–air battery using CoFe2O4 nanoparticles as air–cathode shows the open-circuit voltage (OCV) of 1.47 V. The CoFe2O4 nanoparticles-based Zn–air battery displayed good discharge capacity with 1 mA/cm2 at 1.33 V, 3 mA/cm2 at 1.30 V, and 5 mA/cm2 at 1.28 V, respectively. The CoFe2O4 nanoparticles-based Zn–air battery can work stably and shows better power densities at different current densities, such as 12.45 mW/cm2 at 10 mA/cm2 and 55.91 mW/cm2 at 50 mA/cm2, and the maximum power densities is 142 mW/cm2 at 175 mA/cm2 [Fig. 4(a)]. Furthermore, the CoFe2O4 nanoparticles-based Zn–air battery was made as a commercial product. First, a transparent two opening can was made with an inner diameter of 1 cm and height of 3 cm. Secondly, two matched covers were built for the can, and one of the covers had a spiracle which served as the air collector. Then, the zinc plate and CoFe2O4 nanoparticles-based air–cathode should be fixed. Galvanostatic discharge measurements were conducted at different current densities to study the rechargeable capacity and stability [Fig. 4(b)]. Those Zn-air batteries show a voltage of ∼1.23 V at current density of 3mA/cm2 for 10 h. As expected, the battery fabricated with NiO/CoN PINWs shows a considerably stable charging voltage of 2.3 V and a discharging voltage of 1.25 V at current density of 3 mA/cm2 with the virtually negligible voltage fading for 300 min [Fig. 4(c)]. Additionally, as shown in Fig. 4(d), the self-made products have a beautiful appearance and good performance. The two-series-connected Zn–air batteries were used to power a blue LED (3.0 V) with excellent operation stability (e.g., without obvious change in brightness more than 48 h) [Fig. 4(d)]. The multiple Zn–air batteries with CoFe2O4 nanoparticles as air–cathode can also be used for various applications.
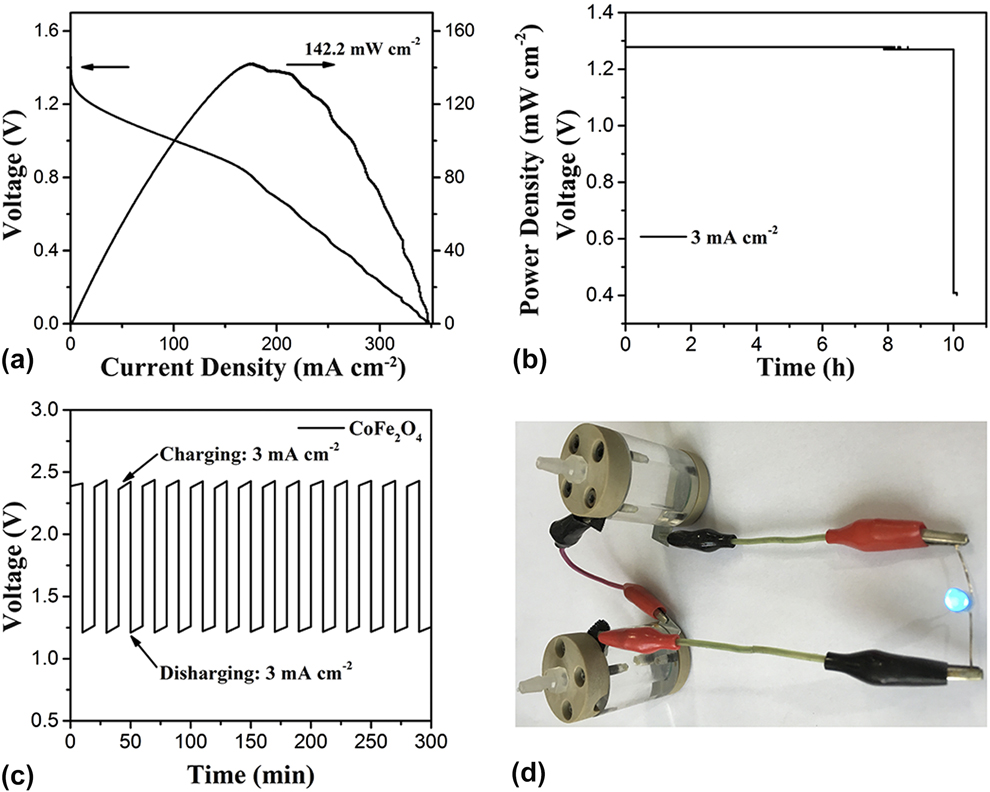
FIG. 4. (a) Discharge voltage curve and the corresponding power density plot of CoFe2O4 nanoparticles tested in the Zn–air battery. (b) Battery cycling test at charging and discharging current density of 3 mA/cm2 in a short interval (10 min per cycle) for CoFe2O4 nanoparticles. (c) Discharge curves of the primary Zn–air battery with CoFe2O4 nanoparticles as the air cathode at 3 mA/cm2. (d) Photograph of a blue LED (∼3.0 V) powered by two-series-connected liquid Zn–air batteries.
IV. CONCLUSIONS
In summary, we report uniform CoFe2O4 nanoparticles with abundant oxygen vacancies and the active crystal face (111) and (110) exposed as efficient bifunctional electrocatalysts for developing high-performance Zn–air batteries. The CoFe2O4 nanoparticles exhibit superior performance for HER in acidic media with an onset potential of 20 mV, an overpotential of 45 mV at current density of 10 mA/cm2, a rapid kinetic reaction with the lower Tafel slope of 35 mV/dec, and remarkable stability at different current densities not less than 100 h. What is more, the CoFe2O4 nanoparticles also displayed a better performance for HER in basic media with the overpotential of 65 mV (at j = 10 mA/cm2). The bifunctional electrocatalytic capacity of CoFe2O4 nanoparticles was confirmed by good performance for ORR. Both the onset potential (0.84 V) and half-wave potential (0.67 V) of the CoFe2O4 nanoparticles for ORR was closer to that of Pt/C (20%), and more importantly, the ORR catalyzed the CoFe2O4 nanoparticles via four electrons processes. Based on the excellent bifunctional electrocatalytic performance, the primary home-made Zn–air batteries were fabricated, showing the OCV of 1.47 V and the maximum power density of 142 mW/cm2. Furthermore, the two-series-connected batteries fabricated by CoFe2O4 nanoparticles can support a LED to work for more than 48 h. The design of uniform nanoparticles with active crystal face exposed for boosting catalysis should also be providing a promising approach for fabricating multifunctional materials for efficient renewable energy applications.
AUTHOR CONTRIBUTIONS
The article was written through contributions of all authors. All authors have given approval for the final version of the article.
ACKNOWLEDGMENTS
The authors acknowledge the support from the National Natural Science Foundation of China (No. 21571089) and the Fundamental Research Funds for the Central Universities (lzujbky-2016-k02, lzujbky-2016-k09, lzujbky-2016-38).