Article contents
Nanowires assembled from iron manganite nanoparticles: Synthesis, characterization, and investigation of electrocatalytic properties for water oxidation reaction
Published online by Cambridge University Press: 15 July 2019
Abstract
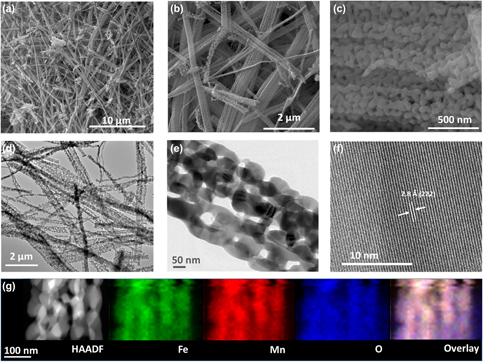
The development of stable and effective earth-abundant metal oxide electrocatalysts is very crucial to improve competence of water electrolysis. In this study, iron manganite (FeMnO3) nanomaterials were synthesized as an affordable electrocatalyst for water oxidation reactions. The structural and chemical properties of FeMnO3 nanomaterials were studied by transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray, X-ray diffraction, X-ray photoelectron spectroscopy, inductively coupled plasma-optical emission spectrometry, and Brunauer–Emmett–Teller analyses. The microscopy analyses show that the synthesized material has wire morphology, and assembly of approximately 70 nm nanocrystallites forms the wires. XRD patterns confirmed the bixbyite structure of FeMnO3. The potential utility of the synthesized FeMnO3 nanowires (NWs) as an electrocatalyst for oxygen evolution reaction (OER) was investigated in alkaline medium. The FeMnO3 NW modified fluorinated tin oxide (FTO) electrodes demonstrated promising OER activity with onset potential of 1.60 V versus reversible hydrogen electrode and overpotential of 600 mV at 10 mA/cm2 catalytic current density. FeMnO3 NW modified FTO electrode was also observed to be stable during long-term constant potential electrolysis. Therefore, this new material can be considered as a cost-effective alternative to noble metal electrocatalysts for water oxidation and other possible catalytic reactions.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 7
- Cited by


