Article contents
The role of alternating current electric field for cell adhesion on 2D and 3D biomimetic scaffolds based on polymer materials and adhesive proteins
Published online by Cambridge University Press: 09 August 2013
Abstract
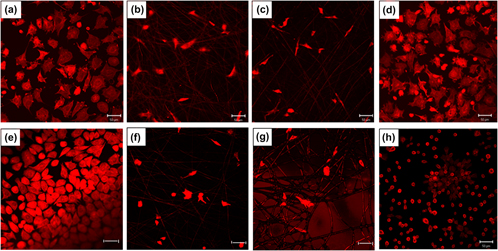
Tissue engineering principles suggest the formation of 3D scaffolds based on polymer fibers and adhesive proteins. These scaffolds aim to mimic the native extracellular matrix and thus providing a favorable environment for cell attachment and proliferation. The application of an electric field (EF) can influence the quantity and the spatial orientation/conformation of adsorbed proteins, which could lead to changes in their functions. We study the influence of alternating current (AC) EF on the adsorption of fibronectin onto poly(etherimide) (PEI) electrospun fiber materials in 3D structures and subsequent cell adhesion. The results are compared with 2D PEI material and glass surface. 3D scaffolds adsorbed a lower amount of fibronectin than 2D film or glass. Application of AC EF with a frequency of 1 Hz decreased the adsorption of fibronectin. Cell adhesion on 3D materials was reduced compared with 2D film and glass. The application of EF with frequencies between 1 and 10 Hz improved cell adhesion on both 2D and 3D materials.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 3
- Cited by


