Introduction
Cardiovascular disease (CVD) is the primary cause of morbidity and mortality(Reference Townsend, Wilson and Bhatnagar1). Preclinical studies suggest that Montmorency tart cherries (MC) can positively impact major risk factors of CVD, activities thought to be related to their phytochemical content(Reference Kelley, Adkins and Laugero2). However, data from human trials are less clear. MC have been shown to reduce systolic blood pressure (SBP) after acute(Reference Keane, George and Constantinou3,Reference Keane, Haskell-Ramsay and Veasey4) , short-term(Reference Desai, Roberts and Bottoms5) and chronic(Reference Chai, Davis and Wright6) intake and to improve vascular dysfunction(Reference Aboo-Bakkar, Fulford and Gates7), reduce LDL-cholesterol(Reference Chai, Davis and Wright6) and oxidised LDL(Reference Johnson, Navaei and Pourafshar8), presumably due to their antioxidant and anti-inflammatory activities(Reference Kelley, Adkins and Laugero2). Notwithstanding, other studies have not shown the benefits of MC on SBP or vascular function(Reference Johnson, Navaei and Pourafshar8–Reference Martin, Burrell and Bopp10). Studies are difficult to interpret with different populations, dosing and durations but we have previously demonstrated that a dosing regimen of 30 ml twice daily (equivalent to 60 ml/d) significantly affected vascular function in mildly hypertensive males(Reference Keane, George and Constantinou3) and middle-aged adults(Reference Keane, Haskell-Ramsay and Veasey4). There is also a lack of understanding for any underlying mechanisms, and to provide mechanistic evidence, the use of metabolomics has become increasingly popular. For example, metabolomics have shown that anthocyanin-rich interventions increase circulating polyphenol metabolites that might account for improvements in vascular function(Reference Istas, Wood and Le Sayec11). Therefore, the aim of this preliminary study was to characterise the effects of MC supplementation with an effective dosage regimen on vascular function with a 4-week intervention and explore if metabolomics can provide mechanistic evidence.
Methods
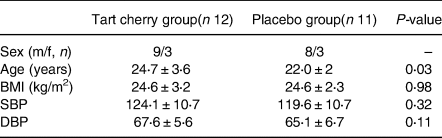
A power calculation was performed for the primary outcome systolic blood pressure. According to the previous study(Reference Keane, George and Constantinou3) and using a population standard deviation of 11 mm Hg at 80 % power and 5 % significance, the minimum number of participants required to allow detection of a difference of 5 mm Hg (clinically relevant outcome) between the responses to the two intervention drinks was estimated to be 12. Twenty-three healthy non-smoking volunteers, who did not regularly consume cherries, use antioxidant supplements or medications, took part in the present randomised (http://randomisation.eu/index.shtml), double-blind, placebo-controlled, parallel study. Participants attended the laboratory on two separate occasions. Participants’ vascular function measures and spot urine samples(Reference Langer, Kennel and Lodge12) were collected following an overnight fast (≥12 h) pre and post 4-week supplementation with either MC concentrate (m/f: 9/3) or an isocaloric placebo (m/f: 8/3). Baseline characteristics are shown in Table 1 and there was a significant difference in age (P = 0⋅03). Participants maintained their habitual diet apart from following a low phenolic diet for 48 h before each laboratory visit. Participants consumed 30 ml of an MC concentrate (CherryActive, UK; containing 36⋅8 mg of anthocyanins (as cyanidin-3 glucoside equivalents using the pH differential colorimetric method) as well as several hydroxycinnamic and hydroxybenzoic acids) diluted in 100 ml of water or the same amount of placebo twice daily, once in the morning and evening. The placebo was a low fruit (<1 %) unsweetened black cherry cordial mixed with ingredients to match MC concentrate for energy and macronutrients (Per 130 ml, energy 102 kcal, carbohydrate 24⋅5 g, protein 1⋅1 g and fat 0 g), colour and taste as described(Reference Keane, George and Constantinou3,Reference Keane, Haskell-Ramsay and Veasey4) . No adverse effects were reported and average self-reported compliance was 86 %. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were ratified by Northumbria University's Research Ethics Committee, and participants provided written informed consent. The trial conforms to CONSORT standards and is registered with ClinicalTrials.gov, identifier NCT04840160.
Table 1. Baseline characteristics of participants
Vascular function was assessed as we have detailed elsewhere(Reference Kimble, Keane and Lodge13) using appropriate commercial devices for blood pressure and heart rate (HR) (M10-IT; Omron Healthcare), pulse wave velocity (PWV) (SphygmoCor CPV system ScanMed Medical, UK) and Digital Volume Pulse (DVP) (PulseTrace PCA 2 with a photoplethysmograph transducer, MicroMedical, UK). Urine samples were prepared for metabolomic analysis as described(Reference Langer, Kennel and Lodge12) following standardisation by refractive index. Hydrophilic interaction liquid chromatography (HILIC) analysis was deemed more appropriate for a more polar biological fluid such as urine. HILIC was conducted using ultrahigh resolution liquid chromatography (UHPLC) and mass spectrometry (MS) (adapted from Langer et al.(Reference Langer, Kennel and Lodge12)) and data acquisition parameters, data processing and data mining information are detailed in the online supplementary methods. Statistical analysis was conducted using SPSS for windows (V24.0: Chicago, IL). A two-way repeated-measures ANOVA analysed the effect of time, treatment and time × treatment interactions for vascular function measures. Baseline characteristics were compared between groups by an unpaired t test. The α level for statistical significance was set at 0⋅05 a priori.
Results and discussion
Despite accumulating evidence for the potential role of cherries in cardiovascular health(Reference Kelley, Adkins and Laugero2), the exact mechanisms have yet to be fully elucidated. Thus, we incorporated a metabolomic approach to explore potential mechanisms. We found MC concentrate to have no significant effect on our indices of vascular function (SBP, diastolic blood pressure, MAP, HR, PWV, DVP-SI or DVP-RI) compared to the placebo after the intervention (P > 0⋅05; Table 2) with only minor changes from baseline, in line with evidence that MC has no effect on vascular function in normotensive individuals(Reference Johnson, Navaei and Pourafshar8–Reference Martin, Burrell and Bopp10,Reference Lynn, Mathew and Moore14) , even those with higher doses(Reference Johnson, Navaei and Pourafshar15) or longer duration(Reference Lynn, Mathew and Moore14,Reference Johnson, Navaei and Pourafshar15) . MC concentrate has been repeatedly shown to result in transient reductions in SBP that return to baseline within 3–4 h(Reference Keane, George and Constantinou3). Since this study investigated vascular function after an overnight fast it is possible that any changes in BP were missed due to the seemingly rapid absorption and/or excretion of tart cherry anthocyanin metabolites(Reference Keane, Bell and Lodge16). It is likely that a combination of dose, duration and population are responsible for the null effects. Durations between 20 days(Reference Desai, Roberts and Bottoms5) to 12 weeks(Reference Chai, Davis and Wright6) have been reported with no association between duration and effect. There is a better likelihood of finding a change in a higher risk population, as in a recent review of factors influencing the effects of anthocyanins, both a higher initial BP and habitual flavonoid intake were identified as relevant determinants of efficacy(Reference Vendrame and Klimis-Zacas17), and these are points to inform future studies with MC concentrate.
Table 2. Influence of Montmorency cherry concentrate on vascular function
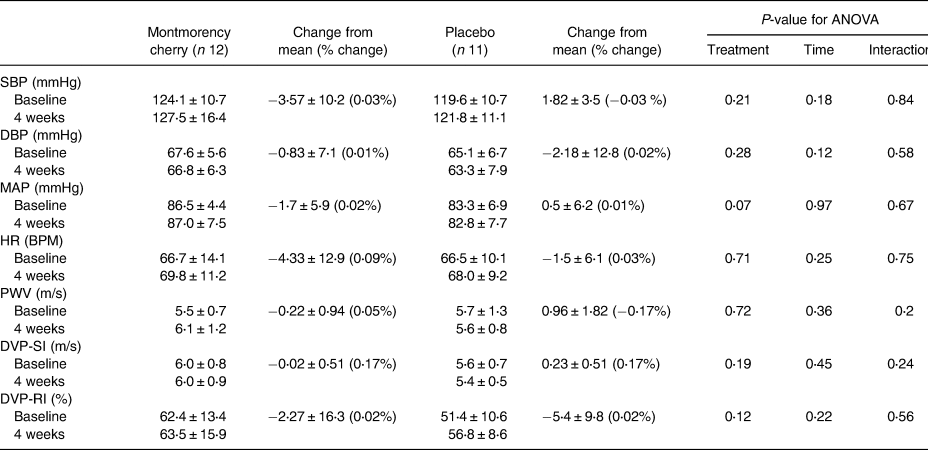
Values are mean ± sd for SBP, DBP, MAP, HR, PWV, DVP-SI, DVP-RI.
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; PWV, pulse wave velocity; DVP-SI, digital volume pulse stiffness index; DVP-RI, digital volume pulse reflection index.
However, we did observe an influence on the urinary metabolome (Fig. 1). There was a good similarity between the metabolome of both treatments at baseline despite limited dietary restrictions and we were able to discriminate post-treatment MC concentrate samples (Fig. 1). Preliminary analysis of the top-ranked discriminatory features found 32 and 18 ion clusters in positive and negative mode, respectively, and Table 3 shows putative matches from these. These included metabolites from tryptophan metabolism (enrichment factor 7⋅1, −log10(p) 1⋅5) including 6-hydroxy melatonin, 5-hydroxyindoleacetate and melatonin in positive mode, while histidine metabolism was prominent in negative ion mode (enrichment factor 15⋅2, −log10(p) 1⋅1) suggesting an influence on tryptophan and histidine pathways even in the absence of changes to vascular function variables. For example, we observed a decrease in urinary excretion of melatonin and 6-hydroxymelatonin. Both tryptophan and melatonin are found in tart cherries(Reference Kelley, Adkins and Laugero2) and have been linked to improvements in sleep following MC supplementation as we have previously shown(Reference Howatson, Bell and Tallent18). Moreover, melatonin and tryptophan metabolites might be beneficial to cardiovascular health because of their antioxidant, anti-inflammatory and antiatherogenic actions(Reference Liu, Chen and Zhong19), suggesting the properties of the indolamine compounds in MC warrant further attention. We also identified an increase in metabolites from the histidine pathway (e.g. histidine, methylated histidine), supporting a study supplementing anthocyanin-rich red wine that resulted in increases in urinary 1-methylhistidine(Reference Jacobs, Fuhrmann and van Dorsten20). We also found matches to expected exogenous metabolites, such as cyanidin glucosides, ferulic acid; however, these were not highly discriminatory, perhaps as they are rapidly cleared(Reference Keane, Bell and Lodge16). Although only preliminary findings, these data provide evidence that intakes of anthocyanin-rich beverages such as MC juice can influence amino acid metabolism and tentatively provide a mechanistic basis for their health effects, although in the present study these changes were not sufficient to elicit a physiological effect.
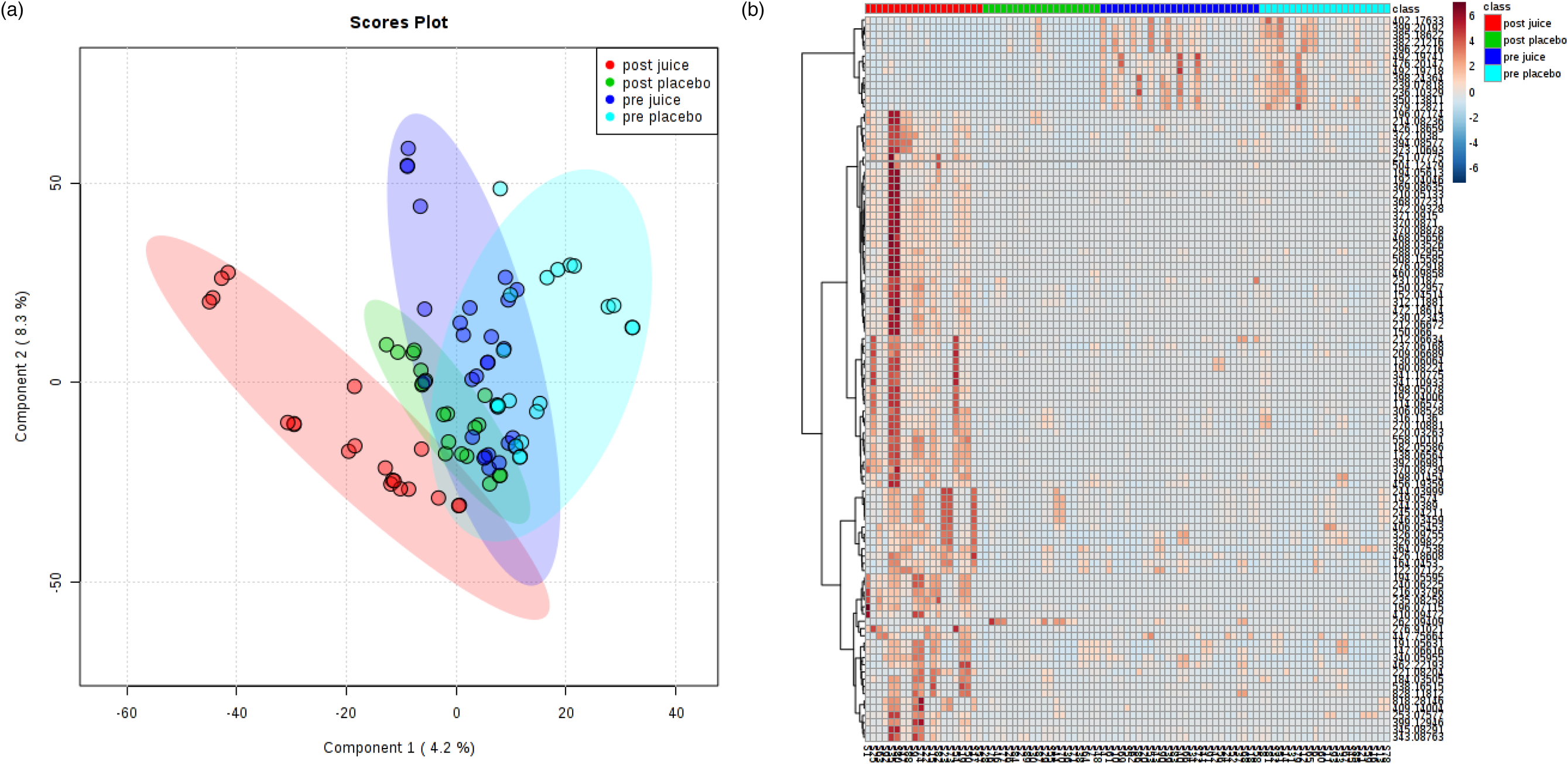
Fig. 1. Multivariate analysis of the effect of cherry juice treatment on the urinary metabolome – (a) shows a score plot of a 4 component partial least squares-discriminant analysis model of all treatments, whilst a heat map of the same data is shown in (b). Negative ion mode data are shown as an example.
Table 3. Putative identifications of highly ranked discriminating metabolites following tart cherry juice supplementation
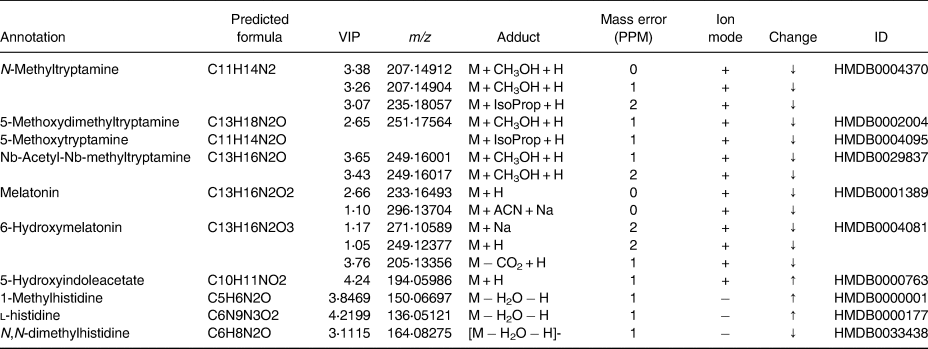
VIP, variable importance in projection.
This study has limitations. We investigated a young, normotensive population, but individuals with higher CVD risk might benefit more from anthocyanin supplementation, or treatment with a longer duration and a higher dosing regimen and a larger number of participants. Notably, the population in the present study was not confounded by medications. Secondly, our dosing regimen was 4-week long and this duration may need to be extended, but importantly the intervention was well tolerated. Furthermore, a crossover study may be more optimal (and especially for metabolomics) as this would reduce inter-individual variation. This study suggests that consumption of MC concentrate for 4 weeks does not influence indices of vascular function in healthy individuals. However, the intervention did induce changes to the urinary metabolome specific to amino acid metabolism and this is the first such report of this effect. These findings have important implications for the physiological effects of tart cherries. Future studies, with larger sample sizes, with higher risk populations and of longer duration are needed to fully elucidate the potential of this intervention but do suggest that metabolomics can be useful to identify metabolic changes indicative of mechanisms of action.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2021.68
Acknowledgments
We would like to thank the Cherry Marketing Institute for funding this research through a joint funded studentship (RK) with Northumbria University and also to the participants in this study. This work was made possible by a grant award to GH by the Michigan Cherry Committee.
L.M. carried out the study as part of an MSc programme and was aided by K.K. G.H. was the principal investigator and conceived the study with J.K.L. K.H. performed sample extraction for metabolomic analysis, which was performed by R.K. and J.K.L. Statistical analysis was performed by R.K. All authors were responsible for the preparation of the manuscript.
Cherry Marketing Institute for funding this research through a joint funded studentship (RK) with Northumbria University.
The authors declare no competing interests to disclose.






