Introduction
A number of clinical interventions may be used to safely manage violent and aggressive behaviour. These may include de-escalation, rapid tranquillisation (RT), control and restraint, and seclusion (supervised confinement). Following unsuccessful de-escalation, RT (the use of medication to rapidly calm the severely agitated or aggressive patient) is recommended as the next intervention.
RT is not underpinned by a strong evidence base. This is partly due to the ethical issues associated with recruiting patients to clinical trials, but also because of a general lack of international consensus regarding the therapeutic aims of this intervention. For example, the highly regarded TREC studies that were undertaken in India and Brazil used a primary outcome of the patient being either tranquil or asleep (Raveendran et al. Reference Raveendran, Tharyan, Alexander and Adams2007; Huf et al. Reference Huf, Coutinho and Adams2007). This is contrary to the UK based definition of RT, which emphasises that the patient should remain conscious throughout and be able to ‘comprehend and respond to spoken messages’ (National Institute of Clinical Excellence, 2005).
There are often high expectations placed on the medicines being selected for RT. The ideal drug should act rapidly, be effective, safe and considering the current politico-economic climate, be cost effective. Few, if any, drugs completely meet all of these criteria, although some are better suited to RT than others.
All service providers should have in place documents that cover the use of RT within their organisations (National Institute of Clinical Excellence, 2005). This is the second of a two-part review examining practice recommendations set out in adult RT documents from trusts providing mental health services in the UK. The first of these (Innes & Iyeke, Reference Innes and Iyeke2011) focussed on the practice of post-RT monitoring. A picture of wide ranging practice was observed and concerning variation identified in what was only one component of the overall RT process. This second article focuses on the drugs, routes and clinical decision-making information influencing their selection and use in RT.
Aim
To identify the drugs recommended, their routes of administration and clinical parameters influencing their use for RT in adult patients across the UK.
Method
Sixty-eight National Health Service (NHS) or Health and Social Care (HSC) trusts providing adult mental health services in the UK were originally contacted between July and August 2010, with a request for copies of their adult RT documents. All mental health trusts in England were contacted together with those trusts identified by a search strategy for Northern Ireland, Scotland and Wales. Those trusts that responded were re-contacted between February and March 2012 to ascertain whether the documents obtained in 2010 were still in use and if not to request updates.
RT documents included policies, guidelines, protocols, procedures, standard operating procedures and algorithms. All were given equal weighting and consideration in this review.
Inclusion and exclusion criteria
Only NHS or HSC Trust adult RT documents confirmed to be in current use, or within their specified review date were included in this review.
Data analysis
Data was extracted by a single reviewer and evidence tables compiled. All data presented were anonymised.
Results
A total of 45 NHS or HSC RT documents met the inclusion criteria for this review. Of these, 38 originated from England, one from Northern Ireland, four from Scotland and two from Wales. Forty-two documents were confirmed to be in current use with another three within their specified review date.
Drugs recommended for use in RT and their respective routes
In total, 16 drugs were recommended for use in RT, via four separate routes of administration: oral, buccal, intramuscular (IM) and intravenous (IV).
The oral route featured in 41 of the 45 documents and a total of 12 drugs was recommended for use. Figure 1 clearly shows that four drugs were recommended most frequently in descending order: lorazepam, haloperidol, olanzapine and risperidone.
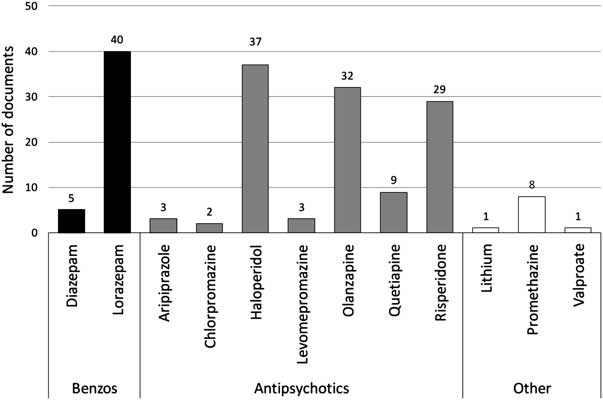
Figure 1 Drugs recommended for oral use in RT
The IM route featured in all documents examined and a total of ten drugs were recommended for use. Figure 2 shows that three drugs were recommended most frequently: lorazepam, olanzapine and haloperidol. Whilst zuclopenthixol acetate also featured frequently, 24 of 32 documents went on to recommend that its use was inappropriate for RT.
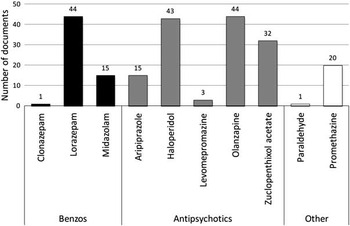
Figure 2 Drugs recommended for IM use in RT
The IV route featured in 19 documents and a total of three drugs were recommended for use. Diazepam was recommended in 13 documents, lorazepam in another two, with two further documents recommending that ‘benzodiazepines’ be used. The antipsychotic haloperidol featured in seven documents.
The buccal route featured in two RT documents with midazolam being the only drug recommended.
Clinical parameters influencing the use of drugs for RT
Clinical decision-making information influencing the selection of drugs for RT was present in nearly all documents examined (n = 44). To enable the reviewer to identify clinical decision-making themes across all RT documents, further analysis was confined to the IM route only, as this applied to all those examined. Regularly occurring clinical decision-making parameters influencing the selection of drugs for RT were identified and examined. Figure 3 illustrates these and their frequency.
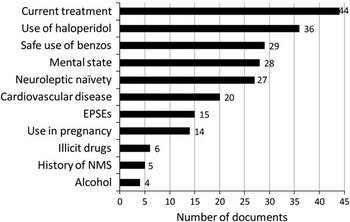
Figure 3 Clinical decision-making parameters influencing IM drug selection in RT
Compatibility with current treatment (n = 44)
This was the most common clinical decision-making parameter appearing in nearly all RT documents. The recommendation that IM olanzapine and IM benzodiazepines should not be administered within an hour of each other proved the most consistent, appearing in 32 documents. Another document recommended the same but with a time interval of two hours and another eight that concurrent administration of IM olanzapine and IM benzodiazepines should simply be avoided. Fifteen documents recommended that other prescribed medications should be considered when selecting medicines for RT, with another six recommending specific care in those already prescribed medicines that could prolong the QT interval. Six documents recommended lorazepam alone in patients already established on antipsychotics; another three recommending this only if the patient was established on high dose antipsychotics, with another recommending lorazepam with or without an antipsychotic. In those already receiving benzodiazepines, three documents recommended the use of antipsychotics, another three haloperidol and another promethazine. Finally, one document recommended that the dose of lorazepam should be reduced by 50% when the patient was being treated with valproate.
Use of IM haloperidol (n = 36)
A variety of instructions and guidance was given but there was no consistent pattern. Three documents recommended haloperidol in those already receiving benzodiazepines, another two recommended that it be used in severe cases and another in those intoxicated with illicit drugs. In contrast, five documents recommended that haloperidol was a last choice drug, with a further nine recommending that it should only be used after a pre-treatment ECG. Eight documents recommended that it should be avoided altogether in neuroleptic naïve patients, another that lower doses should be used in such cases and six that it should be avoided in those with a history of severe extra pyramidal side effects (EPSEs). Finally, one document stated that it should be avoided in those with an unknown response to haloperidol. Seven documents that recommended IM haloperidol did not give any additional or safety guidance about its use.
Safe use of IM benzodiazepines (n = 29)
Fifteen documents stated that benzodiazepines should be avoided in patients with respiratory impairment or disease. Seven documents stated that they should be avoided in those who were benzodiazepine tolerant, with three stating that they should be avoided in those with benzodiazepine hypersensitivity. One document stated that they should be avoided in those who are physically unwell or delirious and two that they should be avoided in those patients who had previously experienced disinhibition with benzodiazepines. Finally, one document recommended that they should be avoided in those intoxicated with alcohol or sedatives. Fifteen documents that recommended IM benzodiazepines gave no specific guidance on their safe use in RT.
Patient's mental state (n = 28)
A range of instructions was given regarding mental state. There was no consistent pattern. Twelve documents recommended that in a non-psychotic context benzodiazepines, or specifically lorazepam, should be used alone. In a psychotic context, lorazepam monotherapy was recommended as first line in two documents whilst seven recommended an antipsychotic with or without benzodiazepines. In ‘moderate’ disturbances two documents recommended the use of olanzapine. In ‘severe’ disturbances one document recommended a combination of haloperidol and lorazepam and another two recommended haloperidol monotherapy. Finally, one document stated that a combination of aripiprazole and lorazepam should be used in an emotionally dominated crisis and olanzapine should be used in a behaviourally dominated crisis. Seventeen documents gave no guidance on how mental state should influence the choice of agent used for RT.
Use of medication in neuroleptic naïvety (n = 27)
Fourteen documents recommended benzodiazepines or specifically lorazepam should be used first line in patients who were neuroleptic naïve. Two documents recommended that antipsychotics should be avoided; a further eight recommending that haloperidol be avoided, whilst one recommended that lower doses of haloperidol should be used. Finally, one document recommended that antipsychotics should be used with caution, with another recommending that olanzapine was the preferred antipsychotic in those who were neuroleptic naïve. Eighteen documents made no mention of tailoring treatment in neuroleptic naïve patients.
Patients with cardiovascular disease (n = 20)
In those with cardiovascular disease, 15 documents recommended benzodiazepines or specifically lorazepam should be used alone, with another recommending that antipsychotic doses should be reduced. A further four documents recommended that antipsychotics should be avoided in patients with compromised cardiovascular function. Twenty-five documents made no reference to tailoring RT treatment in those with cardiovascular disease.
Extrapyramidal side effects or EPSEs (n = 15)
Whilst EPSEs featured heavily in RT documents as a treatment emergent side effect, only 15 included a presence or history of EPSEs as a clinical parameter influencing RT drug selection. Three documents recommended that lorazepam should be used first line in patients with EPSEs, seven recommended haloperidol be avoided and another recommended antipsychotics be avoided all together. Three documents recommended that olanzapine be used as an alternative to other antipsychotics in those with a history of EPSEs. Finally, one document recommended checking whether the patient had a history of EPSEs before prescribing any medicines for RT. Thirty documents made no mention of tailoring treatment in patients with a presence or history of EPSEs.
RT in pregnant patients (n = 14)
Only seven of 45 documents contained a separate section regarding the use of RT in pregnancy. Of these seven documents, four recommended the selection of a psychotropic with a short half-life and also advised that the minimum effective dose be used. One document recommended avoiding lorazepam in the first and third trimesters owing to reports of foetal damage and to avoid haloperidol in the first trimester owing to reported teratogenic effects. The remaining two made no specific recommendations regarding drug selection. Six other documents recommended that zuclopenthixol acetate should not be used, whilst one document actively recommended that lorazepam should be used first line in those who were pregnant.
Impact of concurrent illicit drugs (n = 6)
Information regarding the impact of illicit drugs on medicines for RT was not frequently addressed and when it was, guidance was extremely varied. One document recommended that if a patient had been on illicit drugs then an antipsychotic alone could be used, whilst two became more specific in this situation recommending that olanzapine or haloperidol should be considered. In contrast, three documents stated that lorazepam should be used as monotherapy in this situation.
History of neuroleptic malignant syndrome or NMS (n = 5)
Whilst NMS featured heavily in RT documents as a rare consequence of RT, only five included a previous history as a clinical parameter influencing RT drug selection. Three documents stated that lorazepam should be used in those with a history of NMS, with another stating that antipsychotics should be avoided. Another stated that a history of NMS should be checked before prescribing medication for RT.
Impact of concurrent alcohol use (n = 4)
This scenario was only addressed in four documents. One document stated that benzodiazepines should be avoided in patients intoxicated with alcohol, whilst another opposed this, recommending that lorazepam should be used first line. One document stated that antipsychotics should be avoided if the patient exhibited signs of alcohol withdrawal, with another stating that antipsychotics should be avoided if the patient was intoxicated.
Discussion
Virtually all RT documents included oral psychotropic prescribing as part of the RT pathway, complying with National Institute for Clinical Excellence (2005) guidelines. This is encouraging as it reinforces the concept that RT is a process that begins well before the need for parenteral psychotropics arises. Whilst lorazepam, haloperidol, olanzapine and risperidone stood out as the most commonly recommended oral psychotropics, beyond this there was considerable variation.
The IM medications recommended in RT documents make much more interesting reading. Whilst nearly all recommended lorazepam, olanzapine and haloperidol, other drugs are now being recommended as a result of clinical issues that have arisen with some of these historical gold standard drugs.
The first of these clinical issues is the current national shortage of parenteral lorazepam. Clinicians are being forced to consider alternatives, and many trusts have issued interim guidance or amended their RT documents to reflect possible alternatives. Some have recommended IM midazolam or promethazine whilst others have managed to source and import unlicensed lorazepam from other countries such as the USA.
As stated above, the IM benzodiazepine midazolam is in some places seen as an alternative to lorazepam and its usage appears to be on the increase. Whilst it is supported by some clinical evidence (TREC Collaborative Group, 2003), the use of IM midazolam in RT is an unlicensed indication. It should also be noted that midazolam and lorazepam have different pharmacokinetics, with the former being much faster in its action. Compared to IM lorazepam there is a higher risk of respiratory depression, and this combined with the lack of experience most psychiatric doctors have with it means many are hesitant about using it.
The issue has also been considered by the Care Quality Commission (unpub. newsletter: Mental Health Operations Newsletter for Commissioners and Second Opinion Appointed Doctors (SOADs), June 2011):
‘Given this risk profile, CQC suggests to SOADs that they be especially mindful of midazolam's licensed indications, and of the potential harm that may result post-administration. If certification is being considered, they are likely to wish to satisfy themselves that the provider clinicians are fully aware of the risks, have adequate training in, and equipment for, resuscitation, and that the risks are outweighed by such clear benefits not obtainable from a more mainstream preparation that they can be satisfied that usage is defensible and is that which a reasonable body of medical opinion skilled in mental health, not emergency medicine, would view as appropriate.’
This, together with a National Patient Safety Agency Rapid Response Alert to reduce the risk of midazolam injection overdoses (NPSA, 2008), puts a clear imperative on organisations that advocate its use. Clinicians (doctors, nurses and pharmacists) must have the necessary knowledge, skills and competencies surrounding its use including clinical sequalae and the use of the ‘antidote’ flumazenil.
A significant number of trusts are now using IM promethazine, which is a sedating antihistamine. In some places it is suggested as useful in the benzodiazepine-tolerant patient (Taylor et al. Reference Taylor, Paton and Kapur2012), which may well propagate the belief that it is an alternative when IM lorazepam is unavailable or IM midazolam is an undesirable option. Like midazolam, promethazine is possibly not used widely enough for many psychiatric doctors to be aware of its properties, although its use is backed up by substantial clinical evidence (TREC Collaborative Group, 2003; Alexander et al. Reference Alexander, Tharyan, Adams, John, Mol and Philip2004; Huf et al. Reference Huf, Coutinho and Adams2007; Raveendran et al. Reference Raveendran, Tharyan, Alexander and Adams2007).
The recommendation for a pre-treatment ECG by the manufacturers of haloperidol has presented another clinical challenge to trusts (Janssen-Cilag Ltd, 2011). In spite of this recommendation, virtually all trusts still include haloperidol in their RT documents, with only nine recommending it should be used after an ECG. An important point to note is that the National Institute for Clinical Excellence recommend that all inpatients should have a pre-treatment ECG regardless of what antipsychotic they are prescribed (National Institute for Clinical Excellence, 2009). Inclusion of haloperidol in the RT document is not necessarily a true reflection of how often it is used though. It seems to be heavily restricted in its use and has the second largest number of clinical decision-making parameters allied to it. It will be interesting to see how such recommendations change in the coming years and whether it is superseded in clinical hierarchy by other IM options.
IM olanzapine is now more common than haloperidol in the RT documents used in this review. The primary drawback in the RT scenario is the fact that IM benzodiazepines cannot be given within one hour of IM olanzapine due to serious concerns around safety (Eli Lilly & Co. Ltd, 2012). This proved to be the largest and most consistent clinical decision-making parameter that was identified, with information surrounding this recommendation featuring in 41 of 44 documents. Almost a third of documents included IM aripiprazole, which is regarded as safe when given concurrently with IM benzodiazepines, but as yet doesn't have the cumulative clinical experience of IM olanzapine.
The medium-acting IM antipsychotic zuclopenthixol acetate (Clopixol Acuphase) featured heavily in the RT documents reviewed. However, three quarters of documents that included it then went on to say that it should not be used in RT. The sedative effects of zuclopenthixol acetate begin after about two hours, peak after about 12 hours and can last for up to 72 hours. As such, it has no function in the process of RT, instead having a role to play in the management of serious challenging behaviour in a psychotic patient who has required repeated injections of antipsychotics and/or sedatives. It is understandable that many trusts have used their RT documents to inform clinicians that this drug is unsuitable for RT, although it is concerning that 12 trusts still recommended it as a treatment option.
The IV route featured in less than half the documents examined. This is hardly surprising given the cardiovascular and respiratory risks associated with both IV haloperidol and IV benzodiazepines respectively. The medical risks are probably exacerbated by the challenges of administering IV medications to the active, struggling and restrained patient. It was interesting to see that IV benzodiazepines were clearly more popular than IV haloperidol, possibly because the use of haloperidol via this route is no longer licensed (Janssen-Cilag Ltd, 2011). Anecdotal evidence suggests that the IV route in RT is used extremely rarely.
The buccal route only featured in two documents with midazolam the only drug recommended. Some clinical evidence exists to suggest it may be suitable as a treatment option for RT (Taylor et al. Reference Taylor, Okocha, Paton, Smith and Connolly2008). However, its use in RT is unlicensed and would be subject to the same limitations as the oral route with regard to when it could be administered.
Owing to the complexity of the data we chose to restrict the analysis of clinical decision-making parameters to the IM route. The examination of these offers a clear picture of what trusts consider to be important when prescribing in an RT scenario. In general there were three types of parameters:
1. Parameters related to specific medications, classes of medication or medication side effects.
2. Parameters related to past/current psychiatric diagnoses and treatment.
3. Parameters related to comorbid medical disorders and other health states.
The clinical decision-making parameters in each document are very much dependent on the medications recommended and the type of RT document.
The results of this review have highlighted two concerning issues. The first is the wide variation in advice across different RT documents within the same clinical decision-making parameters; in a small number of cases the advice is even conflicting, for example regarding illicit drug and alcohol use. This leads one to wonder at the existence or rigour of the underlying evidence base for the guidance offered in these areas. The second is the lack of what should surely be essential clinical decision-making parameters in all RT documents. Examples of these include a history of NMS, which was missing from 40 documents, a presence or history of EPSEs which was missing from 30 documents and neuroleptic naïvety which was missing from 18 documents. RT is a situation in which patients will often become exposed to higher doses of parenteral psychotropic drugs. This information is surely essential to ensure that the patient has the best and safest outcome.
What this review has shown is that RT decision-making tools contained within RT documents are complex. A generic RT decision-making tool may look like Figure 4.

Figure 4 An example of a generic RT decision-making tool
This review has a number of limitations. The first is that all types of RT document were given equal weighting and consideration, regardless of their classification. The implications of this are significant because only policies can dictate the practice that must occur, whereas guidelines and other documents of an equivalent standing leave room for clinical discretion. Hence this review points towards the trends suggested by RT documents, it does not offer a definitive picture of actual clinical practice. A second limitation is that all data was extracted by a single reviewer. Whilst this is not unusual for this type of study, it must be acknowledged given the subjective nature of extracting data from documents, especially in relation to clinical decision-making parameters. A third limitation is that analysis of clinical decision-making parameters was limited to the IM route only. Whilst this provided the only available method for comparing all documents, it does mean that any clinical decision-making advice applying specifically to other routes would not have been included.
Conclusions
Practice in this area of psychiatric prescribing may well be changing. At the point of this review, the majority of trusts did include oral medication as part of the RT process, complying with National Institute for Clinical Excellence (2005) guidelines; only ten percent moved straight to parenteral medication.
Many of the drugs used in RT have limitations or relative restrictions on their use. The limitations arise from a number of areas including licence, supply, interactions, side effects, pharmacokinetics and a lack of firm evidence base. Clinical practice is now subject to more external scrutiny than ever before, and many trusts now need to provide assurance that RT is being used in clinically appropriate scenarios and subsequent monitoring takes place post RT.
With the exception of lorazepam, haloperidol and olanzapine, this review shows that there is no consensus over what drugs should be included in an RT decision-making tool. There is a wide variation in advice across different RT documents within the same clinical decision-making parameters and in a small number of cases the advice is even conflicting. In some documents essential clinical decision-making parameters would also appear to be completely missing. These findings are concerning.
RT is a high-risk clinical intervention and it generates all sorts of reactions from patients and clinicians, as it is often used in association with physical restraint. It is virtually impossible to have a one-size-fits-all algorithm for the management of such a diverse range of clinical scenarios. The challenge to academics and clinicians is to rationalise RT, develop consensus guidelines that allow for evidence-based decision-making tools. We hope this review will act as a catalyst in this respect.






