Introduction
Mild cognitive impairment (MCI) is a condition where individuals exhibit a decline in cognitive functioning greater than that of typical aging but remain independent in their activities of daily living (Petersen et al., Reference Petersen, Lopez, Armstrong, Getchius, Ganguli, Gloss, Gronseth, Marson, Pringsheim, Day, Sager, Stevens and Rae-Grant2018). MCI is often characterized as the early, or prodromal, stage of dementia due to Alzheimer’s disease (AD) or other neurodegenerative or cerebrovascular disease processes (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2011). Accurate diagnosis of MCI is critical for early detection and intervention for those at risk for progressive cognitive decline. However, given several diagnostic approaches in use to define MCI, there is unfortunately high variability in its detection across research and clinic samples (Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009; Petersen et al., Reference Petersen, Lopez, Armstrong, Getchius, Ganguli, Gloss, Gronseth, Marson, Pringsheim, Day, Sager, Stevens and Rae-Grant2018).
MCI has typically been defined by the Petersen/Winblad criteria (henceforth referred to as “typical” criteria), which state that an individual has MCI if they are neither cognitively normal nor demented, exhibit cognitive decline from a subjective and objective standpoint, and maintain relatively preserved activities of daily living (Petersen & Morris, Reference Petersen and Morris2005; Petersen, Reference Petersen2004; Winblad et al., Reference Winblad, Palmer, Kivipelto, Jelic, Fratiglioni, Wahlund, Nordberg, Bäckman, Albert, Almkvist, Arai, Basun, Blennow, De Leon, DeCarli, Erkinjuntti, Giacobini, Graff, Hardy, Jack, Jorm, Ritchie, Van Duijn, Visser and Petersen2004). Within this framework, objective cognitive impairment is commonly operationalized as > 1.5 standard deviations (SD) below normative means on at least one neuropsychological measure. The Alzheimer’s Disease Neuroimaging Initiative (ADNI), a large consortium study aimed at validating AD biomarkers and improving treatment trials, uses similar criteria for MCI: subjective memory concerns, impaired score on a paragraph recall test, and intact activities of daily living (Petersen et al., Reference Petersen, Aisen, Beckett, Donohue, Gamst, Harvey, Jack, Jagust, Shaw, Toga, Trojanowski and Weiner2010).
Although efficient, the typical and ADNI criteria for MCI have been critiqued for their tendencies towards misdiagnosis and limited utility in characterizing MCI by type and severity (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014; Edmonds et al., Reference Edmonds, Delano-Wood, Galasko, Salmon, Bondi and Initiative2014; Reference Edmonds, Eppig, Bondi, Leyden, Goodwin, Delano-Wood and McDonald2016). Cluster analyses of neuropsychological test scores from individuals in ADNI’s MCI cohort revealed a “cluster-derived normal” subgroup that closely resembled the cognitively unimpaired comparison group in terms of neuropsychological test performance over time, biomarker profiles, and structural neuroimaging (Edmonds et al., Reference Edmonds, Delano-Wood, Galasko, Salmon, Bondi and Initiative2014; Reference Edmonds, Eppig, Bondi, Leyden, Goodwin, Delano-Wood and McDonald2016; Reference Edmonds, Weigand, Thomas, Eppig, Delano-Wood, Galasko, Salmon and Bondi2018). Similarly, a cluster analysis of test scores from individuals who met typical criteria for MCI in a community-based study also revealed a cluster-derived normal group that did not differ from the standardization group on any neuropsychological measures (Clark et al., Reference Clark, Delano-Wood, Libon, McDonald, Nation, Bangen, Jak, Au, Salmon and Bondi2013). These studies suggest that reliance on subjective report of cognitive concerns and a single objective test measure can lead to both false-positive and false-negative errors as well as limitations in standardization, contributing to inaccurate characterization of cognitive status and misdiagnosis.
In light of the high variability in MCI classification, an actuarial method was developed using neuropsychological test data to classify MCI (Bondi et al., Reference Bondi, Jak, Delano-Wood, Jacobson, Delis and Salmon2008; Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009). These neuropsychological criteria for MCI require an individual to score > 1 SD below age-appropriate norms on two tests within a single cognitive domain, or > 1 SD below age-appropriate norms on one test across at least three cognitive domains (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014; Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009). The use of multiple tests within and across domains to determine diagnosis was designed to balance sensitivity and reliability. Studies comparing different sets of criteria for MCI found that the neuropsychological criteria yielded more dissociable cognitive phenotypes, significant AD biomarker associations, and stable diagnoses compared to the typical or ADNI criteria (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014; Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009). The neuropsychological criteria also better predicted participants’ progression to AD dementia (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014). This research highlighted the benefit of applying more comprehensive neuropsychological methods to diagnostic decision-making and the characterization of MCI. Further, it emphasized concerns that prior MCI studies may have underestimated important biomarker relationships through misclassification of participants. A secondary analysis of the Alzheimer’s Disease Cooperative Study (ADCS) donepezil trial found that removal of the cluster-derived normal participants (i.e., false-positive MCI) unmasked beneficial effects of donepezil in terms of lowering the rate of progression to AD (Edmonds et al., Reference Edmonds, Ard, Edland, Galasko, Salmon and Bondi2017).
Biomarker studies, primarily through ADNI, have investigated the utility of cerebrospinal fluid (CSF) and neuroimaging measures for the early identification of AD and evaluation of clinical trials (Ebenau et al., Reference Ebenau, Pelkmans, Verberk, Verfaillie, van den Bosch, van Leeuwenstijn, Collij, Scheltens, Prins, Barkhof, van Berckel, Teunissen and van der Flier2022; Elman et al., Reference Elman, Panizzon, Gustavson, Franz, Sanderson-Cimino, Lyons and Kremen2020; Hansson et al., Reference Hansson, Seibyl, Stomrud, Zetterberg, Trojanowski, Bittner, Lifke, Corradini, Eichenlaub, Batrla, Buck, Zink, Rabe, Blennow and Shaw2018; Veitch et al., Reference Veitch, Weiner, Aisen, Beckett, Cairns, Green, Harvey, Jack, Jagust, Morris, Petersen, Saykin, Shaw, Toga and Trojanowski2019, Reference Veitch, Weiner, Aisen, Beckett, DeCarli, Green, Harvey, Jack, Jagust, Landau, Morris, Okonkwo, Perrin, Petersen, Rivera‐Mindt, Saykin, Shaw, Toga, Tosun and Trojanowski2022). Updated criteria for the AD continuum, including MCI and preclinical AD, have integrated these biomarkers for the detection and prediction of clinical outcomes (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2011; McKhann et al., Reference McKhann, Knopman, Chertkow, Hyman, Jack, Kawas, Klunk, Koroshetz, Manly, Mayeux, Mohs, Morris, Rossor, Scheltens, Carrillo, Thies, Weintraub and Phelps2011; Sperling et al., Reference Sperling, Aisen, Beckett, Bennett, Craft, Fagan, Iwatsubo, Jack, Kaye, Montine, Park, Reiman, Rowe, Siemers, Stern, Yaffe, Carrillo, Thies, Morrison‐Bogorad, Wagster and Phelps2011). In 2018, the National Institute on Aging - Alzheimer’s Association put forth a biomarker-based research framework in which the AD continuum was defined using an “ATN” classification system based on the presence of β amyloid deposition (A), phosphorylated tau (T), and neurodegeneration (N) (Jack et al., Reference Jack, Bennett, Blennow, Carrillo, Dunn, Haeberlein, Holtzman, Jagust, Jessen, Karlawish, Liu, Molinuevo, Montine, Phelps, Rankin, Rowe, Scheltens, Siemers, Snyder, Sperling, Masliah, Ryan and Silverberg2018). Biomarkers such as CSF β-amyloid 1–42 (Aβ 42), CSF hyper-phosphorylated tau (p-tau), and CSF total tau (t-tau) can appear abnormal several years before the formal diagnosis of dementia and predict progression to AD dementia (Olsson et al., Reference Olsson, Lautner, Andreasson, Öhrfelt, Portelius, Bjerke, Hölttä, Rosén, Olsson, Strobel, Wu, Dakin, Petzold, Blennow and Zetterberg2016; Shaw et al., Reference Shaw, Vanderstichele, Knapik‐Czajka, Clark, Aisen, Petersen, Blennow, Soares, Simon, Lewczuk, Dean, Siemers, Potter, Lee and Trojanowski2009). However, critiques of a biologically-defined AD state that biomarkers alone are not sufficient to define an individual’s position on the AD continuum without clinical input (Dubois et al., Reference Dubois, Villain, Frisoni, Rabinovici, Sabbagh, Cappa, Bejanin, Bombois, Epelbaum, Teichmann, Habert, Nordberg, Blennow, Galasko, Stern, Rowe, Salloway, Schneider, Cummings and Feldman2021). Rather, in clinical settings, diagnosis should consider the presenting clinical phenotype (e.g., an amnestic syndrome), then examine biomarker positivity.
To date, the majority of research studies examining different MCI criteria and phenotypes in conjunction with AD biomarkers have done so in relatively healthy samples with few comorbidities (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014; Edmonds et al., Reference Edmonds, Delano‐Wood, Clark, Jak, Nation, McDonald, Libon, Au, Galasko, Salmon and Bondi2015, Reference Edmonds, Delano-Wood, Galasko, Salmon, Bondi and Initiative2014; Reference Edmonds, Eppig, Bondi, Leyden, Goodwin, Delano-Wood and McDonald2016; Reference Edmonds, Smirnov, Thomas, Graves, Bangen, Delano-Wood, Galasko, Salmon and Bondi2021; Eppig et al., Reference Eppig, Edmonds, Campbell, Sanderson-Cimino, Delano-Wood and Bondi2017; Pommy et al., Reference Pommy, Conant, Butts, Nencka, Wang, Franczak and Glass-Umfleet2023). ADNI, for example, specifically recruited older adults without significant neurological history, psychiatric distress, or high vascular risk (Petersen et al., Reference Petersen, Aisen, Beckett, Donohue, Gamst, Harvey, Jack, Jagust, Shaw, Toga, Trojanowski and Weiner2010). Veteran populations, on the other hand, are typically medically and psychiatrically complicated (e.g., have a high prevalence of post-traumatic stress disorder [PTSD] and history of traumatic brain injury [TBI]) (Carlson et al., Reference Carlson, Kehle, Meis, Greer, MacDonald, Rutks, Sayer, Dobscha and Wilt2011; Greer et al., Reference Greer, Sayer, Spoont, Taylor, Ackland, MacDonald, McKenzie, Rosebush and Wilt2020; Loignon et al., Reference Loignon, Ouellet and Belleville2020; Magruder & Yeager, Reference Magruder and Yeager2009). PTSD has been linked to an increased risk of developing dementia including AD, as well as robust deficits in attention, memory, and processing speed (Desmarais et al., Reference Desmarais, Weidman, Wassef, Bruneau, Friedland, Bajsarowicz, Thibodeau, Herrmann and Nguyen2020; Günak et al., Reference Günak, Billings, Carratu, Marchant, Favarato and Orgeta2020; Scott et al., Reference Scott, Matt, Wrocklage, Crnich, Jordan, Southwick, Krystal and Schweinsburg2015). Similarly, history of TBI has been linked to an increased risk of developing dementia including AD, particularly in Veterans (Gardner et al., Reference Gardner, Bahorik, Kornblith, Allen, Plassman and Yaffe2022; Li et al., Reference Li, Li, Li, Zhang, Zhao, Zhu and Tian2017; Snowden et al., Reference Snowden, Hinde, Reid and Christie2020). Even a history of mild TBI without loss of consciousness in Veterans has been shown to increase the risk for developing dementia (Barnes et al., Reference Barnes, Byers, Gardner, Seal, Boscardin and Yaffe2018). Additionally, Vietnam-era Veterans with histories of TBI have shown elevated levels of CSF p-tau and t-tau, which, in turn, were associated with slower processing speed (Clark et al., Reference Clark, Weigand, Bangen, Thomas, Eglit, Bondi and Delano‐Wood2021). These findings suggest that history of TBI may be associated with pathological brain changes leading to increased risk of dementia.
Given the prevalence of TBI and PTSD in the aging Veteran population, a collaborative study between the Department of Defense and ADNI (DOD-ADNI) was launched to investigate these risk factors for AD in Veterans and their associations with brain AD pathology (Weiner et al., Reference Weiner, Veitch, Hayes, Neylan, Grafman, Aisen, Petersen, Jack, Jagust, Trojanowski, Shaw, Saykin, Green, Harvey, Toga, Friedl, Pacifico, Sheline, Yaffe and Mohlenoff2014). We sought to use the DoD-ADNI database to (1) compare neuropsychological, typical, and ADNI criteria for MCI in a Veteran sample with histories of TBI and/or PTSD in terms of their associations with validated AD biomarkers, and (2) evaluate the effects of TBI and PTSD on diagnosis of MCI by each set of criteria. We predicted that, consistent with past research, the neuropsychological criteria for MCI would show stronger associations with CSF Aβ 42, p-tau181, and t-tau than the typical or ADNI criteria. We also predicted that history of TBI (of any severity) and PTSD symptom severity would both be significant risk factors for MCI by all criteria.
Methods
Data were obtained from the publicly available A Study of Brain Aging in Vietnam War Veterans/DOD-ADNI database (adni.loni.usc.edu). The study is directed by principal investigator Dr Michael Weiner of the San Francisco VA Medical Center and University of California, San Francisco. The overarching goal of the DOD-ADNI study is to investigate the associations between a history of TBI and/or current PTSD and brain AD pathology. The main aims and methods, including participant selection and exclusion criteria, are described in detail elsewhere (Weiner et al., Reference Weiner, Harvey, Hayes, Landau, Aisen, Petersen, Tosun, Veitch, Jack, Decarli, Saykin, Grafman and Neylan2017; Reference Weiner, Veitch, Hayes, Neylan, Grafman, Aisen, Petersen, Jack, Jagust, Trojanowski, Shaw, Saykin, Green, Harvey, Toga, Friedl, Pacifico, Sheline, Yaffe and Mohlenoff2014). Up-to-date information can be found at www.adni-info.org. This research was approved by the Committee on Human Research at the University of California at San Francisco, the San Francisco VA Medical Center Research and Development Committee, and the Department of Defense Human Research Protection Office. Written informed consent was obtained for all study participants. This research was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Participants
Vietnam-era Veterans completed structured clinical interviews including detailed TBI history, self-report assessments, psychodiagnostic assessment, and neuropsychological assessment. 284 Veterans had complete neuropsychological assessment data and 17 participants were excluded from analyses due to missing or inconsistent TBI or PTSD data, resulting in a sample of 267 Veterans. Sociodemographic information is shown in Table 1. The Clinical Dementia Rating (CDR) Dementia Staging Instrument (Morris, Reference Morris1993) and Mini-Mental State Examination (MMSE) (Folstein et al., Reference Folstein, Folstein and McHugh1975) were performed during screening to rule out significant cognitive or functional impairment indicating dementia (i.e., CDR Global ≥ 1, MMSE < 24).
Table 1. Participant characteristics (n = 267)
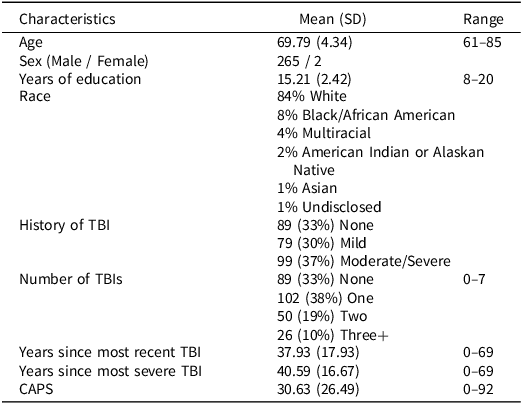
CAPS = Clinician-Administered Posttraumatic Stress Disorders Scale for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, SD = standard deviation, TBI = traumatic brain injury.
TBI and PTSD
Detailed TBI history was obtained using a version of the Ohio State University TBI Identification Method-Interview form. Participants self-reported whether they experienced injuries to the head or neck before, during, and after serving in Vietnam. For each instance of head/neck injury, they provided information about the year of injury, whether they were hospitalized, and the presence and duration of any loss of consciousness (LOC), alteration of consciousness (AOC), or post-traumatic amnesia (PTA). The severity of each injury was classified according to the Veterans Affairs/DoD 2021 Clinical Practice Guidelines (VA/DoD, 2021). An injury was classified as mild if the participant experienced LOC < 30 minutes or AOC or PTA ≤ 24 hours, and moderate/severe if LOC ≥ 30 minutes or AOC or PTA > 24 hours. Current PTSD symptom severity was measured using the Clinician-Administered PTSD Scale for DSM-IV (Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Charney and Keane1998).
Neuropsychological assessment
Memory functioning for the ADNI criteria was assessed using the Wechsler Memory Scale – Revised Logical Memory II subscale (Delayed Paragraph Recall, Paragraph A only). For the neuropsychological and typical criteria, two measures were used for each domain of memory, language, and processing speed/executive functioning, consistent with past literature and tests available in ADNI protocols (Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009). Memory measures included the Rey Auditory Verbal Learning Test (Schmidt, Reference Schmidt1996) delayed recall and recognition. Language measures included animal fluency and the 30-item Boston Naming Test (Kaplan et al., Reference Kaplan, Goodglass and Weintraub1983). Processing speed/executive functioning measures included the Trail Making Test Parts A and B (Reitan, Reference Reitan1956). Raw scores were converted to z-scores using age, sex, and education-adjusted norms (Stricker et al., Reference Stricker, Christianson, Lundt, Alden, Machulda, Fields, Kremers, Jack, Knopman, Mielke and Petersen2021; Weintraub et al., Reference Weintraub, Salmon, Mercaldo, Ferris, Graff-Radford, Chui, Cummings, DeCarli, Foster, Galasko, Peskind, Dietrich, Beekly, Kukull and Morris2009).
Criteria for MCI
The three sets of diagnostic criteria for MCI are defined in Table 2. Subjective memory concerns were assessed from both the participant and their study partner and were coded as yes/no if either reported concerns. For the neuropsychological and typical criteria, MCI was characterized as amnestic-type if the participant scored below cutoff(s) on memory measures, and non-amnestic if the participant scored below cutoff(s) only on measures on language or processing speed/executive functioning.
Table 2. Diagnostic criteria for mild cognitive impairment
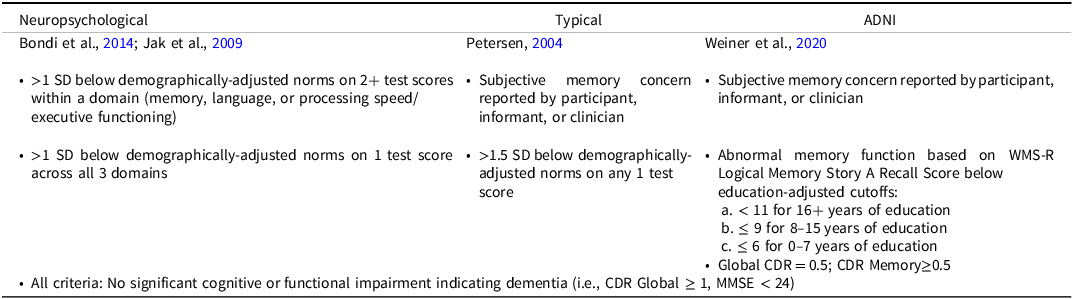
CDR = Clinical Dementia Rating, MMSE = Mini-Mental Status Examination, SD = standard deviation, WMS-R = Wechsler Memory Scale Revised Edition.
Cerebrospinal fluid and genetic markers
A subset (n = 134) of participants completed lumbar puncture to collect CSF samples. CSF levels of p-tau181, t-tau, and Aβ 42 were analyzed using the Roche Elecsys fully automated immunoassay platform and reference LC/MSMS methodology (Kang et al., Reference Kang, Korecka, Figurski, Toledo, Blennow, Zetterberg, Waligorska, Brylska, Fields, Shah, Soares, Dean, Vanderstichele, Petersen, Aisen, Saykin, Weiner, Trojanowski and Shaw2015). Outlier values > 3 SD from the mean for each biomarker were omitted from analyses. Apolipoprotein E (APOE) ε4 positivity was determined by the possession of at least one APOE ε4 allele.
Statistical analyses
Agreement between criteria was assessed using Cohen’s kappa coefficient (κ). Linear multiple regressions adjusting for age and education were conducted to assess associations between MCI status and CSF biomarker by criteria. APOE ε4 positivity was not added as a covariate, as it did not differ between MCI and cognitively normal groups by any criteria. Logistic regressions adjusting for age and education were conducted to simultaneously assess the effects of TBI severity (mild or moderate/severe) and PTSD symptom severity, and potential interactions, on MCI classification by each criteria. Post-hoc analyses assessed the effects of TBI severity and PTSD symptom severity on the subjective and objective components of typical and ADNI criteria.
Results
Identification of MCI
47 (18%) Veterans met neuropsychological criteria, 24 (9%) met typical criteria, and 26 (10%) met ADNI criteria for MCI. The majority of Veterans (201 [75%]) were identified as cognitively normal by all three criteria. Agreement between criteria was poor (Fig. 1). Neuropsychological criteria showed the least agreement with ADNI criteria (κ = 0.11) and minimal agreement with typical criteria (κ = 0.35). Unsurprisingly, typical and ADNI criteria showed the greatest agreement (κ = 0.60) given their common basis of operational definitions. Of those who met neuropsychological criteria for MCI, 31 (66%) were characterized as amnestic MCI (18 single-domain, 13 multi-domain), and 16 (34%) as non-amnestic MCI (13 single-domain, 3 multi-domain). Of those who met typical criteria for MCI, 15 (63%) were characterized as amnestic MCI (7 single-domain, 8 multi-domain), and 9 (37%) as non-amnestic MCI (6 single-domain, 3 multi-domain).
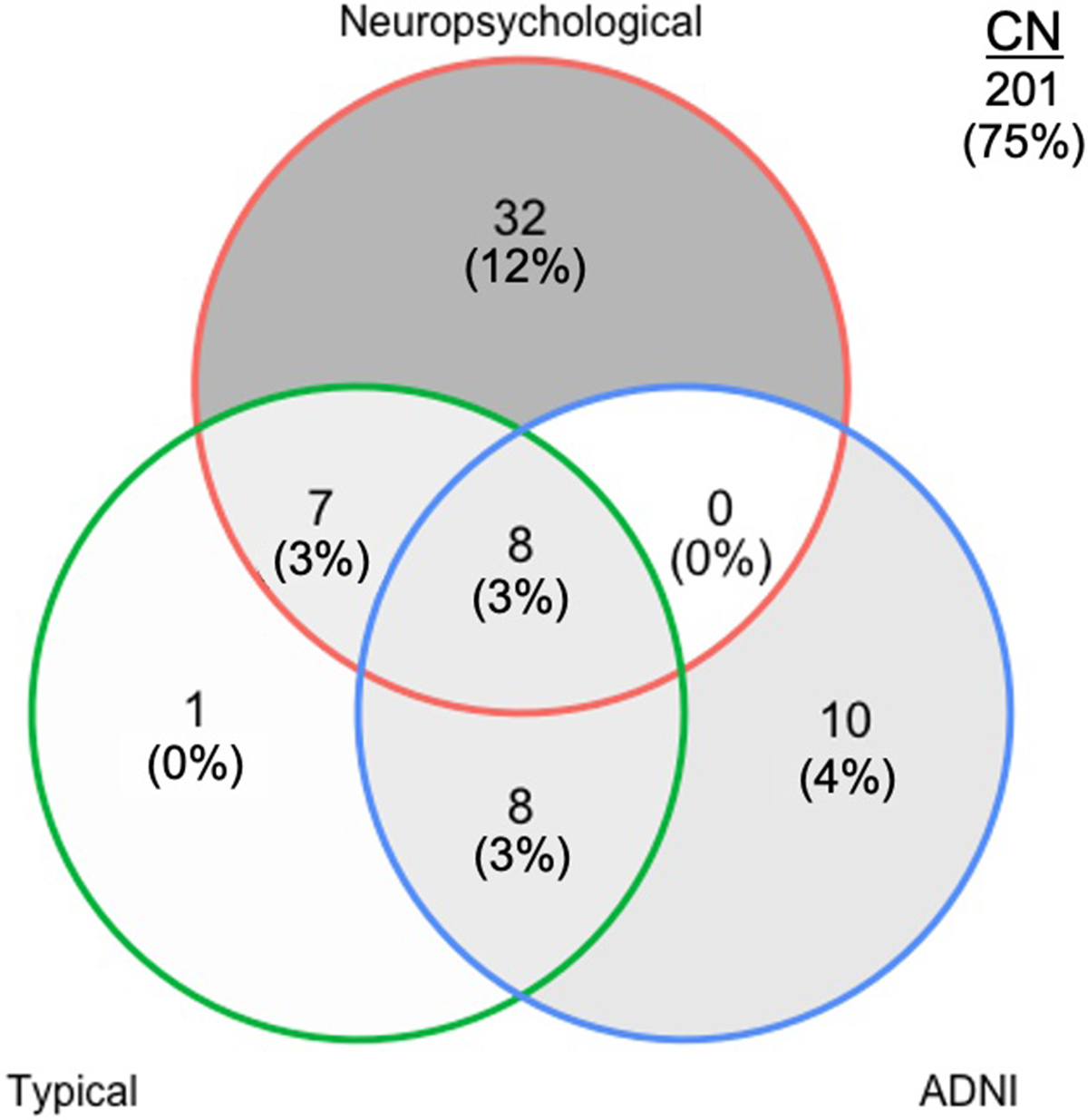
Figure 1. Classification of mild cognitive impairment (MCI) in the DOD-ADNI sample (n = 267) by neuropsychological, typical, and ADNI criteria. CN, cognitively normal; ADNI = Alzheimer’s disease neuroimaging initiative.
Associations with AD biomarkers
MCI diagnosis by neuropsychological criteria was significantly associated with higher p-tau181 and t-tau but was not associated with Aβ 42 (Table 3). MCI diagnosis by typical or ADNI criteria for MCI was not associated with p-tau181, t-tau, or Aβ 42. None of the criteria were associated with p-tau181/Aβ 42 or t-tau/Aβ 42 ratios (all p’s > .05). Subjective memory concerns were not associated with p-tau181, t-tau, or Aβ 42 (all p’s > .15).
Table 3. Multiple linear regressions showing associations between mild cognitive impairment diagnosis by each criteria and cerebrospinal fluid p − tau181, t − tau, and Aβ42 levels after adjusting for age and education
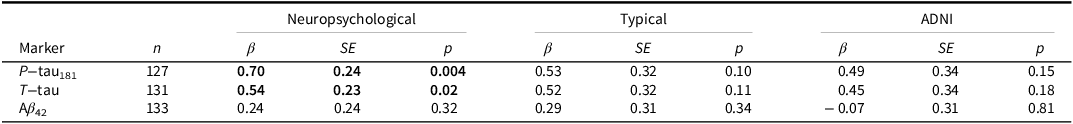
Bolded items indicate p < .05.
Effects of TBI and PTSD on MCI diagnosis
History of mild TBI was not associated with diagnosis of MCI by any criteria (Table 4). History of moderate/severe TBI was associated with MCI by typical criteria and ADNI criteria, but not by neuropsychological criteria. Current PTSD symptom severity was associated with MCI by neuropsychological and ADNI criteria, but not typical criteria (Table 4). Interactions between history of TBI and PTSD symptom severity were not significant (all p’s > .25). Post-hoc analyses found that history of mild TBI (B = 1.06, SE = 0.52, p = .04), history of moderate/severe TBI (B = 1.47, SE = 0.49, p = .003), and PTSD symptom severity (B = 0.53, SE = 0.18, p = .004) were each independently associated with subjective memory concerns. PTSD symptom severity (B = 0.36, SE = 0.14, p = .01), but not history of mild TBI (B = 0.16, SE = 0.34, p = .65) or moderate/severe TBI (B = 0.14, SE = 0.33, p = .68), was associated with scoring > 1.5 SD below demographically adjusted norms on any one test (i.e., objective cognitive impairment by the typical criteria). PTSD symptom severity (B = 0.08, SE = 0.13, p = .52), history of mild TBI (B = 0.21, SE = 0.32, p = .51), and history of moderate/severe TBI (B = 0.22, SE = 0.30, p = .46) were not associated with scoring below cutoff on the Logical Memory Story A recall (i.e., objective memory impairment by the ADNI criteria). In Veterans with history of any TBI, neither time since most recent TBI nor total number of TBIs predicted diagnosis of MCI by any criteria (all p’s > .30).
Table 4. Logistic regressions showing the effects of traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) severity on diagnosis of mild cognitive impairment by each criteria

CAPS = Clinician-Administered Posttraumatic Stress Disorders Scale for Diagnostic and Statistic Manual of Mental Disorders, Fourth Edition.
Bolded items indicate p < .05.
Discussion
We compared neuropsychological, typical, and ADNI criteria for diagnosing MCI in a complex older-adult Veteran sample. Findings showed that identification of MCI was highly variable with evidenced poor agreement between criteria. Neuropsychological criteria classified the largest number of participants as MCI, nearly twice as much as typical or ADNI criteria. Further, neuropsychological criteria, but not typical or ADNI criteria, were significantly associated with AD biomarkers (e.g., higher CSF p-tau181 and t-tau). History of moderate/severe TBI predicted MCI by typical and ADNI criteria. However, history of moderate/severe TBI was only associated with subjective memory concerns and not with objective impairment as defined by the typical or ADNI criteria, suggesting that the relationship between moderate/severe TBI and MCI by the typical or ADNI criteria was driven largely by subjective memory concerns. Mild TBI did not predict MCI by any criteria. PTSD symptom severity predicted MCI by neuropsychological and ADNI criteria.
The variability seen in MCI classifications is consistent with past research in Veterans, which has found that the percentage of individuals classified as MCI can range from roughly 10%–74% depending on the criteria used (Jak et al., Reference Jak, Bondi, Delano-Wood, Wierenga, Corey-Bloom, Salmon and Delis2009). Relatively low percentages were identified in the current Veteran sample because the DoD-ADNI study initially excluded MCI and dementia in their participant recruitment until a later extension of their study aims. Regardless, neuropsychological criteria classified a greater percentage of participants with MCI of both amnestic and non-amnestic types based on objective cognitive data. ADNI criteria focus on both subjective and objective memory functioning to the exclusion of non-amnestic MCI presentations (e.g., dysnomic, dysexecutive). The typical criteria consider cognitive test performances across domains but use a conservative threshold (>1.5 SD below norms). The inclusion of subjective memory concerns in the typical and ADNI criteria likely contributed to their poor agreement with neuropsychological criteria, as subjective cognitive concerns are only weakly associated with objective performance in healthy older adults and those with MCI (Burmester et al., Reference Burmester, Leathem and Merrick2016). Given these approaches, the ADNI criteria are prone to both false-positive diagnostic errors (due to individuals overestimating their cognitive problems) and false-negative diagnostic errors (Edmonds et al., Reference Edmonds, Delano‐Wood, Clark, Jak, Nation, McDonald, Libon, Au, Galasko, Salmon and Bondi2015, Reference Edmonds, Delano-Wood, Galasko, Salmon, Bondi and Initiative2014; Reference Edmonds, Eppig, Bondi, Leyden, Goodwin, Delano-Wood and McDonald2016). The neuropsychological criteria may have reduced false-positives by requiring multiple low scores on objective tests (e.g., lessening base rates of an impaired score in neurologically normal individuals (Brooks et al., Reference Brooks, Iverson and White2007; Palmer et al., Reference Palmer, Boone, Lesser and Wohl1998)). Neuropsychological criteria may have also reduced false-negatives by capturing individuals with cognitive impairment but without subjective concerns (due to limited insight into their cognitive difficulties or use of sufficient compensatory strategies), who likely would have been overlooked by typical and ADNI approaches.
Stronger associations between neuropsychological criteria and CSF biomarkers of p-tau181 and t-tau, compared to other MCI criteria, were consistent with our hypothesis and past research in ADNI (Bondi et al., Reference Bondi, Edmonds, Jak, Clark, Delano-Wood, McDonald, Nation, Libon, Au, Galasko and Salmon2014; Edmonds et al., Reference Edmonds, Eppig, Bondi, Leyden, Goodwin, Delano-Wood and McDonald2016). Pettigrew et al. (Reference Pettigrew, Soldan, Moghekar, Wang, Gross, O’Brien and Albert2015) similarly found that higher CSF p-tau181 and t-tau were associated with poorer episodic memory in cognitively normal older adults, but there was no relationship between Aβ 42 levels and memory. Generally, increased tau burden has been associated with poorer semantic and episodic memory in older adults with and without MCI (Nathan et al., Reference Nathan, Lim, Abbott, Galluzzi, Marizzoni, Babiloni, Albani, Bartres-Faz, Didic, Farotti, Parnetti, Salvadori, Müller, Forloni, Girtler, Hensch, Jovicich, Leeuwis, Marra, Molinuevo, Nobili, Pariente, Payoux, Ranjeva, Rolandi, Rossini, Schönknecht, Soricelli, Tsolaki, Visser, Wiltfang, Richardson, Bordet, Blin and Frisoni2017; Pelgrim et al., Reference Pelgrim, Beran, Twait, Geerlings and Vonk2021; Reijs et al., Reference Reijs, Ramakers, Köhler, Teunissen, Koel-Simmelink, Nathan, Tsolaki, Wahlund, Waldemar, Hausner, Vandenberghe, Johannsen, Blackwell, Vanderstichele, Verhey and Visser2017). In addition, subjective memory concerns, a key component of the typical and ADNI criteria, were not associated with AD biomarkers. These results suggest that the use of objective cognitive measurements is most aligned with the biological definition of AD while demonstrating clinically relevant cognitive impairment.
A strength of our study was the concurrent analysis of the effects of TBI and PTSD on MCI given their frequent prevalence and co-occurrence in Veterans (Carlson et al., Reference Carlson, Kehle, Meis, Greer, MacDonald, Rutks, Sayer, Dobscha and Wilt2011). While several studies have investigated the effects of head injury/TBI as a risk factor for dementia including AD (Gardner et al., Reference Gardner, Bahorik, Kornblith, Allen, Plassman and Yaffe2022; Li et al., Reference Li, Li, Li, Zhang, Zhao, Zhu and Tian2017), few have examined risk for developing MCI. LoBue et al. (Reference LoBue, Denney, Hynan, Rossetti, Lacritz, Hart, Womack, Woon, Cullum and Abisambra2016) found that in the National Alzheimer’s Coordinating Center database, history of TBI with LOC was associated with increased odds of MCI and earlier diagnosis of MCI, though both effects were substantially attenuated by history of depression and demographic factors (e.g., sex, race). Li et al. (Reference Li, Risacher, McAllister and Saykin2016) similarly found that within the ADNI cohort, history of TBI was associated with earlier age of onset of cognitive impairment, as measured by criteria closely resembling ADNI’s MCI criteria. However, they did not examine the impact of psychiatric factors on diagnosis.
We found that history of moderate/severe, but not mild, TBI was associated with MCI by typical and ADNI criteria. Secondary analyses found that history of mild or moderate/severe TBI was related to subjective concerns but not objective impairment as defined by the typical or ADNI criteria, which is consistent with research showing that the majority of military service members who endorsed subjective memory concerns following mild to severe TBI scored within normal ranges on objective memory tests (French et al., Reference French, Lange and Brickell2014). Subjective cognitive concerns following TBI may bias diagnostic decision-making towards MCI even in the absence of objective cognitive impairment.
We also found that PTSD symptom severity predicted MCI by neuropsychological and ADNI criteria. Studies have found relationships between PTSD severity and increased incidence of MCI in World Trade Center Responders, and increased rate of MCI and dementia diagnoses in Veterans (Bhattarai et al., Reference Bhattarai, Oehlert, Multon and Sumerall2019; Clouston et al., Reference Clouston, Diminich, Kotov, Pietrzak, Richards, Spiro, Deri, Carr, Yang, Gandy, Sano, Bromet and Luft2019). PTSD severity has also been shown to play a larger role than mild TBI in the relationship between subjective cognitive concerns and objective cognitive performance (Mattson et al., Reference Mattson, Nelson, Sponheim and Disner2019). Therefore, PTSD is an important and treatable risk factor to consider when assessing Veterans for neurocognitive disorders.
The DOD-ADNI investigators recently examined prevalence of MCI in the same Veteran cohort as the current study (Weiner et al., Reference Weiner, Harvey, Landau, Veitch, Neylan, Grafman, Aisen, Petersen, Jack, Tosun, Shaw, Trojanowski, Saykin, Hayes and De Carli2023). They identified MCI in a larger proportion of participants (51 out of 289; 18%) and concluded that TBI and PTSD both predicted diagnosis of MCI. The difference in findings is likely attributable to their operationalization of MCI, which differed from our study’s use of the ADNI criteria by adding a telephone screening assessment and ultimately relying on clinician judgment. Although clinician judgment could theoretically integrate subjective and objective information with more nuance than following strict criteria, data-driven diagnoses of MCI can still outperform clinician/consensus diagnoses in terms of capturing individuals with abnormal AD biomarkers that are likely to progress to dementia (Edmonds et al., Reference Edmonds, Smirnov, Thomas, Graves, Bangen, Delano-Wood, Galasko, Salmon and Bondi2021). Weiner and colleagues’ operationalization of TBI also differed from our study such that they categorized participants into groups (e.g., control, TBI, PTSD, or TBI & PTSD) rather than analyzing TBI and PTSD dimensionally.
Limitations of the current study include recruitment by the DOD-ADNI that favored cognitively unimpaired adults, which limited our statistical power in comparing MCI and cognitively unimpaired groups. Additionally, given the sample demographics (e.g., predominantly White, older male Veterans), the generalizability of these results is limited and may not extend to younger, racially/ethnically diverse, female, or civilian groups. Furthermore, this study used a coarse measure of subjective memory concerns, and future research should examine more comprehensive, structured measures of subjective cognition that encompass domains other than memory, such as the Everyday Cognition Test (Farias et al., Reference Farias, Mungas, Harvey, Simmons, Reed and DeCarli2011). TBI characteristics were obtained by retrospective self-report and TBI-related biomarkers such as CSF neurofilament light were not available for analysis. Finally, this study used a cross-sectional analysis that cannot speak to causal relationships between TBI, PTSD, cognition, and AD pathology. Future studies should examine the stability of MCI diagnosis (e.g., reversion rates, progression to AD) by each criteria in the DOD-ADNI sample, expand to other biomarkers of neurodegeneration, and assess potential moderating genetic factors such as APOE ε4 carrier status.
In summary, neuropsychological criteria for diagnosis of MCI appear to be a more sensitive and reliable method of diagnosis that is aligned with biological definitions of early AD compared to typical or ADNI criteria. Diagnostic methods for neurocognitive disorders should incorporate comprehensive neuropsychological methods, particularly for populations with complex medical and psychiatric comorbidities.
Data availability statement
Data from the DOD-ADNI study are publicly available under ADNI’s Data Sharing and Publication policy at adni.loni.usc.edu.
Acknowledgements
Data used in preparation of this article were obtained from the Department of Defense Alzheimer’s Disease Neuroimaging Initiative (DOD-ADNI) database (adni.loni.usc.edu). As such, the investigators within the DOD-ADNI contributed to the design and implementation of the study and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. We thank all the veterans for their generous participation in this DOD-ADNI study.
Authorship contribution
M. T. Ly contributed to study design, conducted data analysis and interpretation, and drafted the manuscript. J. Adler contributed to data interpretation, drafting, and revisions of the manuscript. A. Ton Loy contributed to drafting and revisions of the manuscript. E. Edmonds contributed to study design, data interpretation, and revisions of the manuscript. M. Bondi contributed to study design, data interpretation, and revisions of the manuscript. L. Delano-Wood contributed to study design, data interpretation, and revisions of the manuscript.
Funding statement
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD-ADNI (Department of Defense award numbers W81XWH-12-2-0012, W81XWH-13-1-0259, and W81XWH-14-1-0462). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. DOD-ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles.
Competing interests
Dr. Bondi receives royalties from Oxford University Press. The remaining authors have no disclosures to report.







