There are places in the world in which it is good to live; there always have been. The Santa Elina rock shelter, in Central Brazil, is one such place. It was recurrently occupied by several groups of hunter-gatherers from the Late Pleistocene to the Late Holocene (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017; Vilhena-Vialou Reference Vilhena-Vialou and Vilhena-Vialou2005, Reference Vilhena-Vialou and Vialou2011; Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019). They left behind almost a thousand paintings and drawings, in many colors, representing images from geometric signs to highly realistic figurative animals and humans (Vialou Reference Vialou and Vilhena-Vialou2005)—making this one of the most impressive rock art sites in Brazil. These hunter-gatherers also left remains of their lithic industry, adornments, braided fibers, basketry, and many ecofacts, such as plant remains in a perfect state of preservation (Vilhena-Vialou Reference Vilhena-Vialou and Vilhena-Vialou2005). Many extinct megafaunal remains were found in the lower levels, before 10,000 cal BP, some in clear association with material culture (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017; Vilhena-Vialou Reference Vilhena-Vialou, Miotti, Salemme and Flegenheimer2003, Reference Vilhena-Vialou and Vialou2011).
The site, thoroughly excavated, yielded dates ranging from around 1500 to 27,000 cal BP (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017). Exceptionally good preservation of plant remains is restricted to the late Holocene levels. Combustion structures and dispersed charcoal occur throughout the stratigraphy. Some of the hearths, large and dense, were reused several times (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Vilhena-Vialou Reference Vilhena-Vialou and Vilhena-Vialou2005).
The possibility of people living in South America before 12,000 BP remains contentious. Nevertheless, research in various fields has provided growing evidence of the existence of pre-Terminal Pleistocene sites and of multiple independent, geographically uneven migration routes into South America (cf. Araujo and Ferreira Reference Araujo and Ferreira1996; Boëda et al. Reference Boëda, Clemente-Conte, Fontugne, Lahaye, Pino, Felice, Guidon, Hoeltz, Lourdeau, Pagli, Pessis, Viana, da Costa and Douville2014; Bueno et al. Reference Bueno, Dias and Steele2013; Dillehay et al. Reference Dillehay, Zamora-Ramírez, Pino, Collins, Rossen and Pino-Navarro2008; Moreno-Mayar et al. Reference Moreno-Mayar, Vinner, de Barros Damgaard, de la Fuente, Chan, Spence, Allentoft, Vimala, Racimo, Pinotti, Rasmussen, Margaryan, Orbegozo, Mylopotamitaki, Wooller, Bataille, Becerra-Valdivia, Chivall, Comeskey, Devièse, Grayson, George, Harry, Alexandersen, Primeau, Erlandson, Rodrigues-Carvalho, Reis, Bastos, Cybulski, Vullo, Morello, Vilar, Wells, Gregersen, Hansen, Lynnerup, Lahr, Kjær, Strauss, Alfonso-Durruty, Salas, Schroeder, Higham, Malhi, Rasic, Souza, Santos, Malaspinas, Sikora, Nielsen, Song, Meltzer and Willerslev2018). The antiquity of the Santa Elina shelter was carefully analyzed in a recent article, in which the authors argued for the crucial significance of the site in understanding the earliest period of known prehistoric settlement in South America (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017).
Anthracology may contribute to this debate, because the analysis of charcoal assemblages may allow us to discriminate between fires of natural or anthropogenic origin (Scheel-Ybert Reference Scheel-Ybert and Smith2018). In addition, it provides data on vegetation changes and aspects of plant uses that are key to understanding past ways of life.
In this article, we present the latest results of anthracological analysis of dispersed and concentrated charcoal from throughout the stratigraphic record of Santa Elina. We aim to reconstruct the landscape, vegetation, and climate over the several thousand years of occupation and to provide information regarding firewood management.
Environmental and Archaeological Backgrounds
The Santa Elina rock shelter is situated in Mato Grosso state, Central Brazil (15°27′28″S, 56°46′93″W, 290 m asl), on the southeastern side of the Serra das Araras range, at the base of a rocky massif, on the northeastern face of one of its ridges (Figure 1). The region is located at the confluence of two major river basins: Paraná/Paraguay and Tocantins/Araguaia. Cuiabá River, a main tributary of the Paraguay River, runs through the range about 30 km east of the shelter (Aubry Reference Aubry and Vilhena-Vialou2005; Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017).
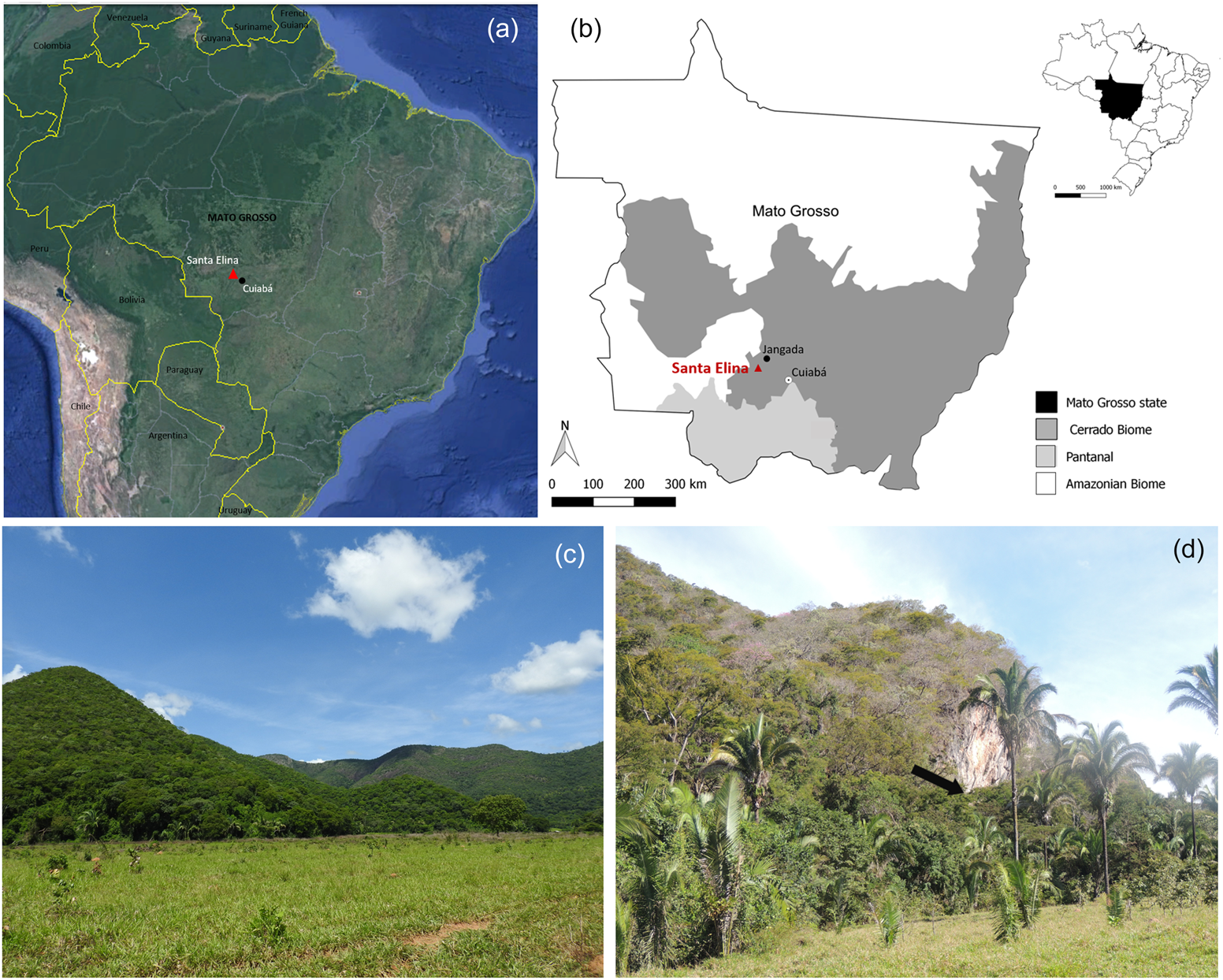
Figure 1. Location and general view of the study area. (a) General location of the site (base map adapted from Google Earth); (b) location of the site in Brazil, the Mato Grosso State, and distribution of the major regional biomes; (c) general overview of the Serra das Araras in the studied region; (d) view of the location of the Santa Elina rock shelter (at the base of the rock wall that appears in the center of the photograph, as indicated by the arrow). Map and photographs by Caroline Bachelet. (Color online)
The climate is warm tropical (Aw in the Köppen classification), with a well-marked dry season from May to September. This region is located within the cerrado biome, characterized by a mosaic of plant formations distributed according to climate, substrate, topography, and human action. It comprises park and gramineous-woody savannas (campo limpo, campo sujo, campo rupestre), wooded savannas (cerrado stricto sensu, veredas), and forest formations (deciduous, semi-deciduous, and riparian forests, as well as cerradão—which is a typical local dry forest; Coutinho Reference Coutinho and Klein2002; Rizzini Reference Rizzini1997).
The geological, climatic, and environmental characteristics of the Serra das Araras make for a unique landscape. Thick vegetation thrives thanks to a variety of subsoils, developed because of the regional hydrological network (Ceccantini Reference Ceccantini and Vilhena-Vialou2005). The local landscape comprises patches of deciduous and semi-deciduous forests, cerrado, riparian forests, and anthropogenic areas (deforested areas, pastures, habitations). The rocky massif where the shelter is situated is presently surrounded by deciduous and semi-deciduous forests, whereas cerrado formations and riparian forests occur in the adjoining plains. Deciduous and semi-deciduous forests are characterized by taxa such as Aspidosperma sp., Inga edulis, Enterolobium sp., Qualea sp., and the like; cerrado formations are characterized by Aspidosperma macrocarpon, Curatella americana, Casearia sylvestris, Guazuma ulmifolia, and so on; riparian forests are characterized by Tapirira obtusa, Hirtella gracilipes, Anadenanthera colubrina, Ficus guianensis, and the like (Ceccantini Reference Ceccantini and Vilhena-Vialou2005).
Several species belonging to these formations can be used for food, medicine, crafts, construction, charcoal production, and firewood (Lorenzi Reference Lorenzi2002; Lorenzi and Matos Reference Lorenzi and Matos2008). The diversity of plant resources—fruits, flowers, seeds, and underground storage organs—available throughout the year makes this area attractive not only to humans but also to animals, several of which were hunted (Pacheco Reference Pacheco2008).
The shelter, formed between two precambrian dolomitic limestone walls, is between 3 and 4 m wide and about 20 m long. The floor slopes slightly to the north and east. The space between the two walls is filled with sand and fallen blocks. To the south is a large rock wall 50 m high, 60 m long, and with a negative inclination of 70°. The shelter is fully protected from the weather, providing favorable conditions for human settlement that were largely exploited (Vilhena-Vialou Reference Vilhena-Vialou and Vilhena-Vialou2005).
This site was excavated between 1984 and 2004 in two contiguous areas totaling 80 m2 (Sector East/Sector West). Several human groups are thought to have occupied the shelter in succession. Most were hunter-gatherers, but cultivators did at least visit the site in more recent times, as attested by rare ceramic sherds and a maize cob retrieved from the surface layer and dated to 400 ± 50 BP (Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019). Three main periods of occupation were identified in the stratigraphy (Pacheco Reference Pacheco2008; Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017; Vilhena-Vialou Reference Vilhena-Vialou, Miotti, Salemme and Flegenheimer2003, Reference Vilhena-Vialou and Vilhena-Vialou2005, Reference Vilhena-Vialou and Vialou2011; Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019):
(1) The upper archaeological layers (stratigraphic unit I: SU-I), or Holocene levels, exhibit a detailed chronology between 7000 and 1500 cal BP of a series of uninterrupted and well-stratified occupations. The sediments, fine and powdery, are formed primarily of ash. The preservation of plant remains is exceptional. There are many wooden posts, probably the remnants of habitational structures and other uses. Numerous combustion features are rich in charcoal and sometimes contain fruits and other plant remains, stone tools, and adornments. Lithic artifacts and colorants are very frequently found; the latter have been associated with rock art and body paintings. Animal remains, although not very conspicuous, include fish, reptiles, amphibians, birds, mammals, and invertebrates.
(2) The intermediate archaeological layers (stratigraphic unit II: SU-II), or Pleistocene/Holocene transition levels, are dated from 12,000 to 7000 cal BP. The sediments are sandy; noncharred plant remains are rare. The material culture is characterized by a rich lithic industry, combustion features, and dispersed charcoal. Animal remains are similar to those of SU-I in frequency but include extinct faunal remains of Glossotherium in the lower levels.
(3) The lower archaeological layers (stratigraphic unit III: SU-III), or Pleistocene levels, are dated to the Late Pleistocene (22,500 ± 500 BP–23,120 ± 260 BP). The sediments are sandy and stony; plant remains are scarce. Many Glossotherium megafauna remains were retrieved, frequently in direct association with lithic artifacts. The material culture is characterized by lithic and microlithic industries; in addition, there are two modified osteoderms, probably adornments. Plant remains are restricted to few dispersed charcoal fragments. Animal remains are fewer than in SU-II and much less diverse, suggesting that human occupation during this period was sparse and sporadic.
Previous Studies on Plant Remains
Exceptionally well-preserved plant remains were retrieved from the more recent levels at Sector West, dated between about 6000 and 1500 BP. In addition to charcoal, wood and bamboo posts, leaves, fibers, basketry, artifacts, fruits, and seeds were found. Several studies were aimed at proposing interpretations of plants uses; most were conducted by G. Ceccantini and collaborators (e.g., Ceccantini Reference Ceccantini2002; Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005; Ceccantini and Gussella Reference Ceccantini and Gussella2001; Gussela Reference Gussella2003).
Wooden posts occur in all stratigraphic levels of SU-I (Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005). Nearly a hundred of them were found in situ, some measuring up to 60 cm. Two parallel rows of posts were distributed on each side of the shelter over a length of 11 m. Posts in both rows are situated in two different stratigraphic levels: one double alignment is between SU-I-1b and SU-I-2, and another one is between SU-I-2 and SU-I-3. In addition to a few sparse posts, a third alignment occurs perpendicularly to the first ones, “closing” them at their western end; this third alignment is situated between SU-I-1b and SU-I-2 (Kamase Reference Kamase and Vilhena-Vialou2005; Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019). The analysis of 59 posts identified 21 taxa in 15 dicotyledonous families (Astronium sp., Tapirira sp. [Anacardiaceae], Adenocalymna sp., Tabebuia sp. [Bignoniaceae], Protium sp., Burseraceae indet [Burseraceae], Cecropia sp. [Cecropiaceae], Terminalia sp. [Combretaceae], Sloanea sp. [Elaeocarpaceae], Apuleia sp., Hymenaea sp., Inga sp., Myrocarpus sp. [Leguminosae], Trichilia sp. [Meliaceae], Ficus sp. [Moraceae], Myrtaceae indet, Randia armata [Rubiaceae], Magonia pubescens, Talisia sp. [Sapindaceae], Guazuma ulmifolia, Pterygota sp. [Sterculiaceae]), and two genera of bamboo (Guadua sp., cf. Merostachys). Guadua is by far the most common material used (found in 39% of the posts), followed by Astronium (8.5%) and Tabebuia (5%). All other taxa are found in only one or two posts. Both high- (Hymenaea, Astronium, Apuleia, Tabebuia, Terminalia, Talisia) and low-density woods (Ficus, Protium, Tapirira, Pterygota, Trichilia, Guadua) were used. Ecological features suggest that wood was mostly obtained from deciduous and semi-deciduous forests (Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005). Vilhena-Vialou and Vialou (Reference Vilhena-Vialou and Vialou2019) interpret the aligned posts as being part of a shelter, possibly covered with palm leaves; some posts might also have supported structures such as platforms or shelves.
Many leaves/leaflets of palms and other species were excavated from SU-I (Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019). Leaves of a Marantaceae appeared isolated, sometimes fragmented but often stacked, and also as the wrappings of small packages of unknown contents that were tied with braided fibers (Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005).
A variety of artifacts produced from plant materials were found throughout the living space. Most were concentrated in SU-I-1a and SU-I-1b (dated from ca. 2000–1500 cal BP), but they occur up to SU-I-3 (ca. 4000 cal BP; Blanchot and Amenomori Reference Blanchot, Amenomori and Vilhena-Vialou2005). Basketry, penile sheaths, sandals, braided fibers, cords, strings, complex knots (some typical of festive armbands), and indeterminate artifacts were produced from palm leaves. Histological analysis of 24 of these artifacts revealed a predominant use of Attalea eichleri (42% of artifacts) and Astrocaryum sp. (12.5%; Gussela Reference Gussella2003). Fourteen of 50 fragments of braided strings and possible adornments and necklaces were identified. They were produced from palm (Bactris glaucescens), Hibiscus sp., and bromeliads fibers (Blanchot and Amenomori Reference Blanchot, Amenomori and Vilhena-Vialou2005; Vilhena-Vialou and Vialou Reference Vilhena-Vialou and Vialou2019). Analysis of weaving, braiding, twisting, folding, and knotting techniques suggested that these artifacts might be associated with Bororo material culture (Taveira Reference Taveira and Vilhena-Vialou2005).
Many coils of unbraided fibers were retrieved from all SU-I stratigraphic levels in different units. Initially interpreted as possible raw material for braided artifacts, histological analysis determined that the analyzed samples were actually woody liana stems of the genus Aristolochia. These forest plants, with a strong and unpleasant scent, are widely cited in the literature for their various properties. Despite their toxicity, Aristolochia species are used as medicine in various parts of the world for many purposes, they have attested antiophidic properties and are used as repellents and amulets against snakes, and they have ritual uses (Ceccantini and Gussella Reference Ceccantini and Gussella2001). This plant may have been used for any or several of these purposes on this site. The abundance of Aristolochia stems suggests that it was culturally important for the site dwellers for at least the last 6,000 years.
A resin fragment of Hymenaea courbaril (SU-I-1b) may also have been used medicinally. This resin, extracted from the bark or fruit of the tree, is traditionally used to treat various disorders, including pain, bone fractures, diarrhea, parasites, bronchitis, inflammation, and snake bites (Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005).
Well-preserved fruits and seeds occurred in various contexts at SU-I: dispersed in sediments, concentrated in features, and associated with hearths, they were the subject of two separate analyses. All samples come from unsystematic collections retrieved manually during the excavations. Gussela (Reference Gussella2003) analyzed 624 samples of plant material collected in several field seasons between 1986 and 2002. All fruits and seeds larger than 0.5 cm and presenting diagnostic characteristics were analyzed (n = 7,814). Scheel-Ybert and Solari (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) analyzed 70 fruits and seeds, along with other noncharred plant remains collected during the 1995 excavation.
Both studies yielded quite similar results. Gussela (Reference Gussella2003) identified nine taxa from seven different families (Arecaceae, Anacardiaceae, Apocynaceae, Combretaceae, Humiriaceae, Leguminosae, Phytolaccaceae); Scheel-Ybert and Solari (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) identified 12 taxa, also from seven families (Arecaceae, Apocynaceae, Bignoniaceae, Humiriaceae, Lecythidaceae, Leguminosae, Sterculiaceae; Figure 2). Variations in the relative proportions between the different analyses may be due to unsystematic sampling—this also explains the few taxa identified. Quantitative results should thus be taken with caution; there may be an underrepresentation of smaller and less conspicuous fruits/seeds and an overrepresentation of those most valued by field archaeologists.
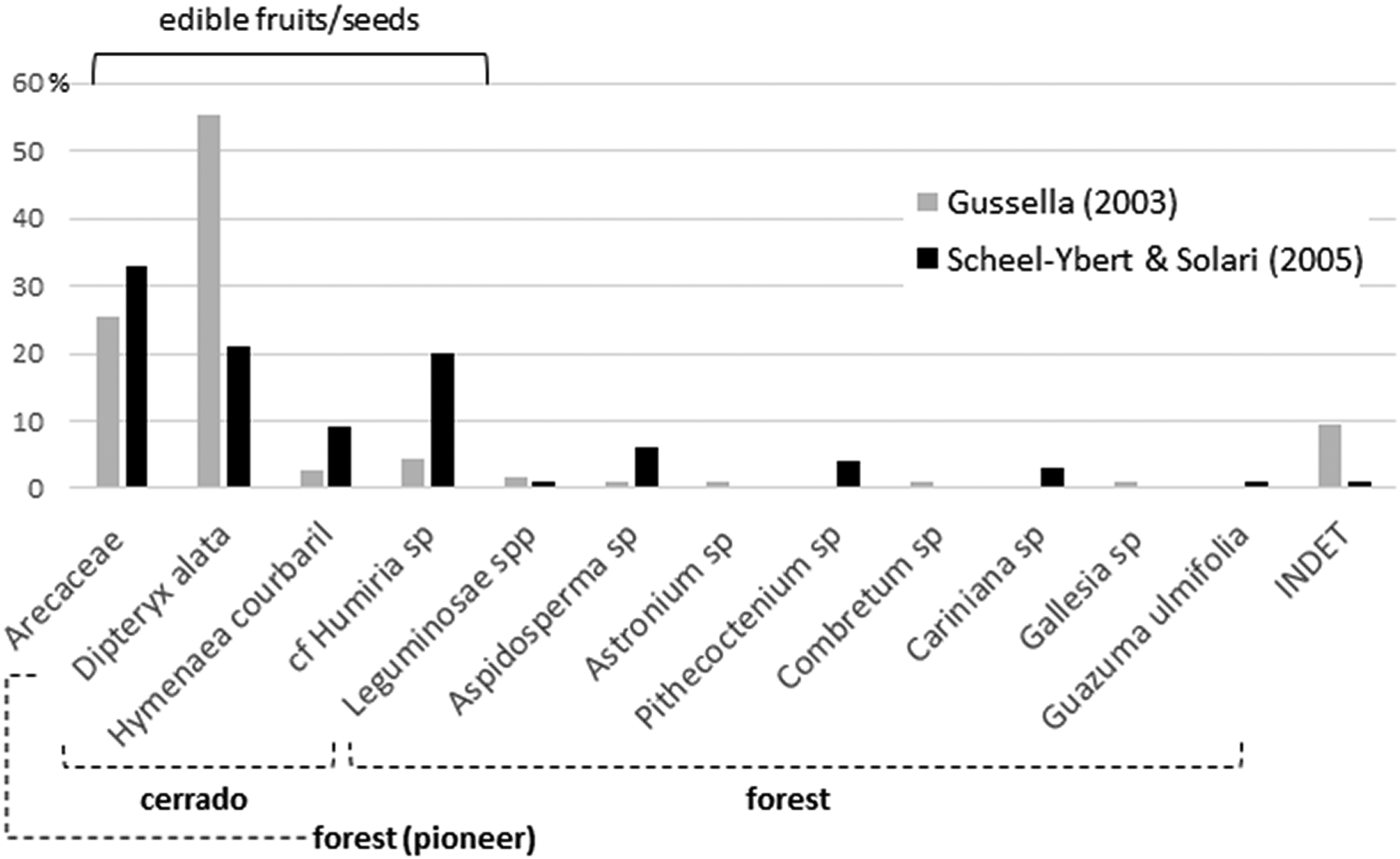
Figure 2. Synthesis of the carpological analyses performed by Gussella (Reference Gussella2003) and Scheel-Ybert and Solari (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) for the Santa Elina rock shelter. Among the palms, Scheel-Ybert and Solari (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) identified three species (21% Orbignya oleifera/babaçu, 9% Acrocomia aculeata/bocaiúva, and 3% Scheelea phalerata/acuri), while Gussella (Reference Gussella2003) left all remains at the family level; the histogram was constructed on family level to allow comparability.
Only the five most frequently observed taxa are common to both studies: Arecaceae (palms), Dipteryx alata, Hymenaea courbaril, cf. Humiria sp., and Aspidosperma sp. With the exception of the last taxon, they all produce edible fruits or seeds. In addition, a noncharred wood fragment retrieved in 1995 attests to the existence in the area of Spondias sp. (Anacardiaceae), the fleshy fruits of which are highly appreciated still today (Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005).
In both samples, legumes and palms are the most important plants, both in species diversity and in number of specimens. These two families account for most of the food items identified, especially D. alata, H. courbaril, and palms. Scheel-Ybert and Solari (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) identified three palm species (Orbignya oleifera, Acrocomia aculeata, and Scheelea phalerata; Figure 2), all of which produce edible fruits/seeds and palm hearts, as well as fibers or leaves widely used in basketry. S. phalerata leaves are appreciated as shelter coverings, and A. aculeata and O. oleifera have medicinal properties.
Among the other carporemains is Pithecoctenium crucigerum, a woody liana typical of forest edges and clearings, whose large fruits are currently used as toys or for crafting. Cariniana sp. and Albizia niopoides are typical forest species whose fruits are appreciated by animals. Astronium sp., Combretum sp., Gallesia sp., D. alata, and H. courbaril are recognized for their medicinal properties (Gussela Reference Gussella2003; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005).
Gussela's (Reference Gussella2003) analysis, covering the entire SU-I, demonstrates a wide temporal and spatial distribution of fruits and seeds. Before 4000 yrs BP, the record shows a smaller number of species, with increased diversity after about 2000 yrs BP. Scheel-Ybert and Solari's (Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) analysis also verified a higher diversity after about 2000 yrs BP. Nevertheless, higher diversity does not necessarily reflect variation in the patterns of plants uses, because it could be a result of collection and preservation biases.
Materials and Methods
Standard archaeological methods enabled the careful removal of anthropogenic sediments over different field seasons. Charcoal samples were collected following two different methods: (1) charcoal and carporemains concentrated in combustion features and associated deposits were sampled by hand picking and dry sieving (all field seasons), and (2) dispersed macroremains in the sediments were systematically sampled by dry sieving (1990, 1995, and 1997 seasons; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005). All the plant material (charcoal, fruits/seeds, wood, leaves, artifacts, and so on) was sorted in the field and subsequently forwarded to different specialists. Anthracological analyses were carried out at ISEM UMR 5554, Institut des Sciences de l'Evolution de Montpellier (Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005) and in the Museu Nacional in Rio de Janeiro, reported in this article.
In the laboratory, large samples of charcoal from SU-I were subsampled, whereas samples from SU-II and SU-III were analyzed in their entirety. All charcoal fragments larger than 3 mm were analyzed in each sample/subsample. Charcoal pieces were manually split according to the three fundamental wood sections (transverse, tangential longitudinal, and radial longitudinal) and analyzed under reflected light brightfield/darkfield microscopes. Taxonomic determination was performed by referring to the specialized literature (e.g., Détienne and Jacquet Reference Détienne and Jacquet1983; Fedalto et al. Reference Fedalto, Mendes and Coradin1989; Mainieri and Chimelo Reference Mainieri and Chimelo1989) and to the reference collection of the Laboratory of Archaeobotany and Landscape of the Museu Nacional in Rio de Janeiro (Scheel-Ybert Reference Scheel-Ybert2016). Wood and charcoal anatomy databases, especially InsideWood (2004; Wheeler Reference Wheeler2011) and Anthrakos (Scheel-Ybert et al. Reference Scheel-Ybert, Boyadjian, Mateus, Paranaguá, Puerto, Korstanje and Inda2014), were consulted as well.
In this article, we compare the following four datasets:
(1) SU-I-1 to SU-I-3: 59 samples of dispersed charcoal from four archaeological layers of Sector West (squares 32–40 A–D); spanning about 4000–1500 cal BP (results published in Scheel-Ybert and Solari [Reference Scheel-Ybert, Solari and Vilhena-Vialou2005] and Bachelet and Scheel-Ybert [Reference Bachelet and Scheel-Ybert2017])
(2) SU-I-4 to SU-II-1: five samples of dispersed charcoal from two archaeological layers of Sector East (squares 23–24 A–B); dated between about 8000 and 7000 cal BP (unpublished analysis performed by Rita Scheel-Ybert)
(3) SU-II-2 concentrated (SU-II-2_C): eight samples from combustion structures of Sector East (squares 21–23 A; results published in Bachelet and Scheel-Ybert [Reference Bachelet and Scheel-Ybert2017] but reinterpreted here); the F7 combustion structure was directly dated to 9340 ± 70 BP
(4) SU-II-2 to SU-III-4: 40 samples of dispersed charcoal from two archaeological layers of Sector East (squares 20–24 A–Z and 26–28 A–C); dated at around 11,000 and 27,000 cal BP, respectively (unpublished analysis performed by Caroline Bachelet)
Results
The four datasets compared in this article span the occupation of Santa Elina. We analyzed 4,695 charcoal pieces in 113 samples, covering all the surface and stratigraphy of the site (Table 1). Approximately 15% of these pieces correspond to non-identifiable pieces (knots, bark, tiny stems, poorly preserved, or vitrified fragments), 80% were taxonomically identified to the family or genus level (n = 3,758), and 5% remain undetermined. Fragments of charred palm endocarps were identified in several samples from SU-I and SU-II. Those fragments are not considered here because their numbers are not significant due to prior separation of most of the carpological remains from these samples. The importance of palms as food items has already been well established by previous studies (Gussela Reference Gussella2003; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005).
Table 1. Characterization of the Samples Analyzed in the Present Study.
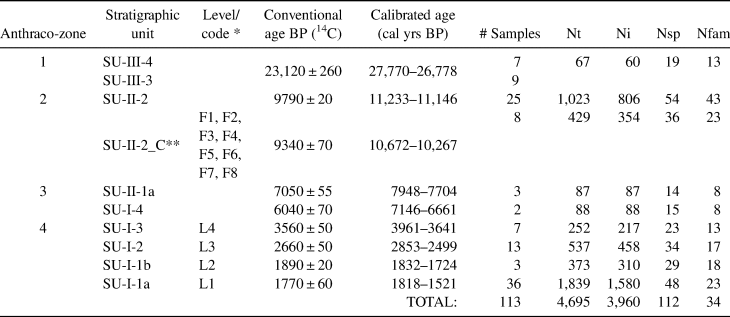
Note: Definition of anthraco-zones, stratigraphic units, chronology, number of subsamples, total number of charcoal pieces analyzed (Nt), number of charcoal pieces that could be determined (Ni), number of identified taxa (Nsp), and number of families (Nfam).
* Code adopted by Bachelet and Scheel-Ybert (Reference Bachelet and Scheel-Ybert2017) in correspondence to stratigraphic unit code.
** Concentrated charcoal from SU-II-2: synthesized results for all the combustion features analyzed in Bachelet and Scheel-Ybert (Reference Bachelet and Scheel-Ybert2017); the date provided was obtained directly from feature F7.
Dataset SU-II-2_C comprises the conjugation of anthracological results from concentrated charcoal in eight combustion features (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017). Concentrated charcoal remains usually represent a brief period of use and contain the remnants of only one or a few charring events, thus forming species-poor assemblages that provide only paleoethnobotanical information (Scheel-Ybert Reference Scheel-Ybert and Smith2018). Nevertheless, the analysis of multiple-use features (such as F4 and F8 are likely to be; Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017) or of a large number of features or both may allow paleoecological interpretations and the identification of firewood acquisition practices as well (cf. Scheel-Ybert Reference Scheel-Ybert and Smith2018). In the present case, the large numbers of features and of charcoal pieces analyzed, associated with high species diversity and with results that are consistent with the dispersed charcoal, help ensure that our interpretation is reliable.
In our analysis of the entire sample, we identified 34 botanical families—32 dicotyledons and 2 monocotyledons—and 84 genera, of which only one is a monocotyledon; 28 taxa remain undetermined. The Leguminosae family is dominant, both in the number of taxa (n = 41) and in the frequency of charcoal pieces (51% of all identified fragments). Subfamilies Caesalpinioideae and Mimosoideae are in the large majority (10 genera each, compared to only 3 from Papilionoideae); 28 Leguminosae types remain identified to the family level.
The Leguminosae family is followed, in frequency of charcoal pieces, by the Anacardiaceae (10%), Bignoniaceae (6%), Rubiaceae (5%), Euphorbiaceae (5%), Apocynaceae (1%), Sapotaceae (1%), and Annonaceae (1%); besides a high frequency of Bambusoideae (bamboos) and Arecaceae (palm stems). In the number of taxa, the Leguminosae family is followed by Rubiaceae (n = 10), Anacardiaceae (n = 6), Euphorbiaceae (n = 4), Sapotaceae (n = 3), and Malvaceae (n = 3). The most important dicotyledon genera are Anadenanthera spp. (Leguminosae, 15% of the charcoal pieces), Parapiptadenia sp. (Leguminosae, 9%), Astronium sp. (Anacardiaceae, 7%), Inga spp. (Leguminosae, 6%), Tabebuia spp. (Bignoniaceae, 6%), Croton sp. (Euphorbiaceae, 5%), Cassia spp. (Leguminosae, 4%), and Piptadenia sp. (Leguminosae, 3%).
The analysis of the anthracological results allowed the definition of four “anthraco-zones” (Figures 3 and 4; Table 1). They do not match the abovementioned four datasets but are ordered chronologically.
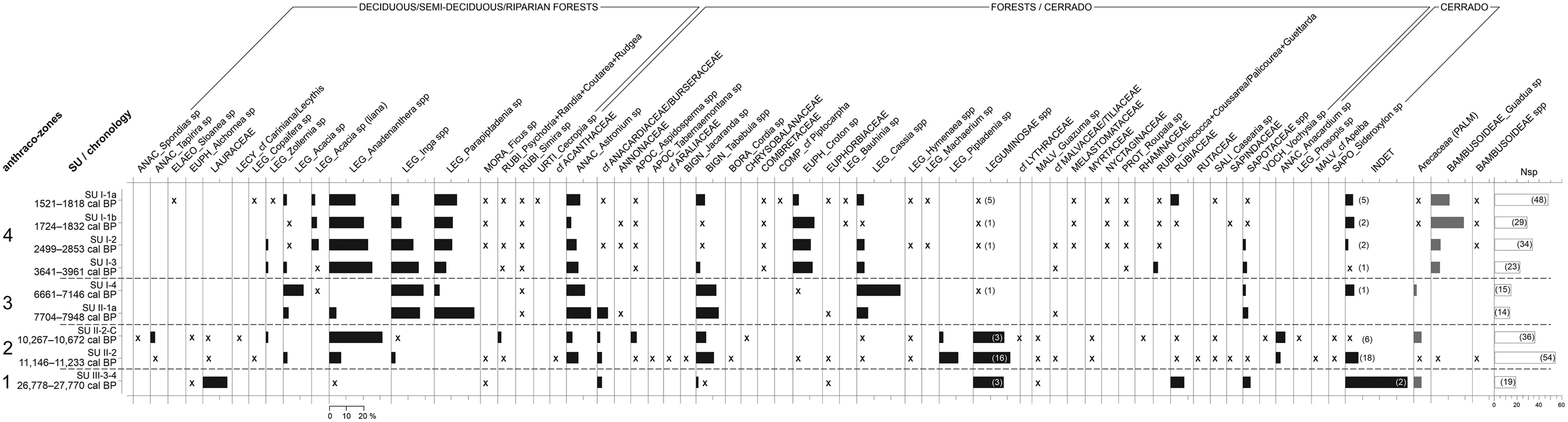
Figure 3. Anthracological diagram for the Santa Elina rock shelter. The presence of taxa with frequencies under 2% is indicated with an “x.” The number of anatomical types for Leguminosae and for undetermined types is given in parentheses. The number of taxa identified at each level (Nsp) is given in the last histogram. Histograms in gray indicate taxa that were not included in the anthracological sum. (A full-size version of this diagram is included as Supplemental Figure 1.)
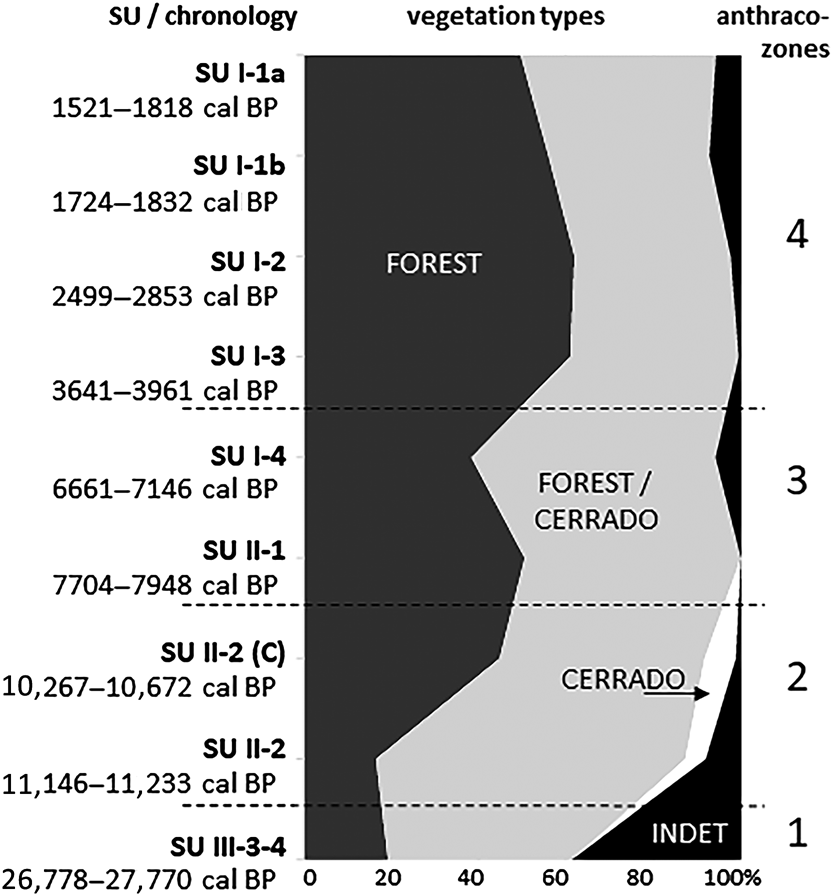
Figure 4. Summary charcoal diagram for the Santa Elina rock shelter.
Anthraco-Zone 1 (AZ-1)
This zone corresponds to the earlier occupation of this site, dated at about 27,000 cal BP (SU-III-3, SU-III-4). A very low plant diversity (19 taxa from 13 families) reflects the low number of fragments available, the lowest in the entire sample. The small charcoal sample can be explained by taphonomic issues (poor preservation) and also by less intensive fire use, possibly associated with the episodic occupation of the site during this early period.
AZ-1 is dominated by the families Leguminosae, Lauraceae, Rubiaceae, and Sapotaceae (Figures 3 and 5). There are no relevant genera in AZ-1; most taxa are only identified at the family level.
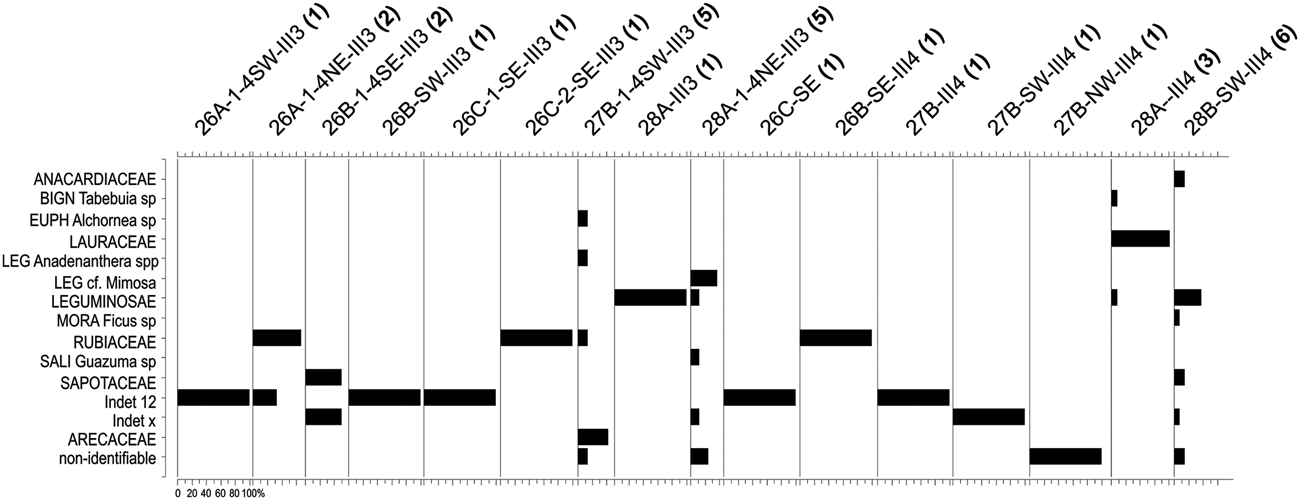
Figure 5. Histograms indicating the taxonomic composition of the Unit I samples analyzed from the Santa Elina rock shelter. The number of taxa in each sample is given in parentheses after the sample code.
The vegetation is very poorly represented in this zone. This is due both to the insufficient sample number, which does not reliably represent the surrounding landscape (cf. Chabal Reference Chabal1997; Scheel-Ybert Reference Scheel-Ybert and Smith2018), and to the large number of undetermined specimens (ca. 40% of the sample). Nevertheless, the high frequency of Lauraceae associated with the presence of Alchornea (a typical riparian forest plant) does point to a forested environment.
Anthraco-Zone 2 (AZ-2)
This zone corresponds to dispersed and concentrated charcoal from mid SU-II, dated between about 11,000 and 10,000 cal BP (SU-II-2, SU-II-2_C). High plant diversity (36–54 taxa per level, 23–43 families) indicates a reliable representation of the surrounding vegetation (cf. Chabal Reference Chabal1997; Scheel-Ybert Reference Scheel-Ybert2004, Reference Scheel-Ybert and Smith2018).
The assemblage is dominated by the families Leguminosae (both in frequency and in the number of taxa), Anacardiaceae, Bignoniaceae, Rubiaceae, Malvaceae, Salicaceae, and Apocynaceae. Anadenanthera spp. is the most frequent taxa; Tabebuia spp., Piptadenia sp., and Astronium sp. also appear in significant concentrations. These taxa indicate forest vegetation surrounding the site. Concurrently, taxa strictly associated with cerrado formations (Anacardium sp., Prosopis sp., cf. Apeiba, and Sideroxylon sp.) attest to their prevalence in the landscape.
Anthraco-Zone 3 (AZ-3)
This zone corresponds to the end of SU-II and the beginning of SU-I, dated from about 8000–7000 cal BP (SU-II-1a, SU-I-4). Despite a drastic change in the nature of the archaeological sediments between SU-II-1a and SU-I-4 (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017), the composition of the anthracological assemblage is very similar.
The drastic reduction in plant diversity (14–15 taxa per level, 8 families each) is probably associated with the small number of charcoal pieces available for analysis. As with AZ-1, the fewer charcoal pieces in the sediments might be due to taphonomic issues or less intense human occupation during this period.
The assemblage is dominated by the families Leguminosae, Anacardiaceae, and Bignoniaceae. This zone marks a clear reduction in Anadenanthera spp. and in unidentified Leguminosae, with greater concentrations of Cassia spp., Inga spp., Parapiptadenia sp., Acacia sp., and Astronium sp., and slightly more Tabebuia spp.
All the taxa identified are characteristic of the deciduous/semi-deciduous/riparian forest or may occur both in the forest and in cerrado formations. Most of the charcoal pieces analyzed exhibit growth rings, pointing to a seasonal climate, with the alternation of dry and rainy seasons. A high proportion of fragments bearing thick bark corroborates the occurrence of a severe dry season or possibly an adaptation to fire or both.
Anthraco-Zone 4 (AZ-4)
This zone corresponds to the more recent occupations analyzed in Sector West, dated to between about 4000 and 1500 cal BP (SU-I-1a, SU-I-1b, SU-I-2, SU-I-3). Plant diversity is very high, especially in SU-I-1a (23–48 taxa per level, 13–23 families). The assemblage is dominated by the Leguminosae family, followed by Anacardiaceae, Euphorbiaceae, Rubiaceae, and Bignoniaceae. Anadenanthera spp. is the most frequent taxa in all levels, comprising 21% of all identified fragments in AZ-4. It is followed by Parapiptadenia sp., Inga spp., Astronium sp., Croton sp., and Tabebuia spp. Charred bamboo pieces (Guadua sp.) are extremely frequent. Growth rings and thick barks remain abundant. All taxa identified are characteristic of the deciduous/semi-deciduous/riparian forest or may occur both in the forest and in cerrado formations; there is a discrete augmentation in forest taxa compared to AZ-3. The data analyzed here suggest that during this period the shelter was surrounded by a forested environment similar to the present one.
Sample validity was tested through saturation curves (Figure 6). In all samples but AZ-1, the curves tend to stabilize, indicating that at least most of the species used in each level are represented in the charcoal samples. Even in AZ-3, where less than a hundred charcoal pieces were analyzed in each level, saturation curves suggest that the vegetation is reasonably well represented. In evaluating these curves, one must consider that species-area/saturation curves constructed for tropical environments hardly attain asymptotes (Scheel-Ybert Reference Scheel-Ybert2002). This is true both in phytosociological (e.g., Assunção and Felfili Reference Assunção and Felfili2004; Felfili and Felfili Reference Felfili and Felfili2001; Neri et al. Reference Neri, Meira Neto, da Silva, Martins and Batista2007) and paleoecological studies (e.g., Ledru Reference Ledru1991; Scheel-Ybert Reference Scheel-Ybert2000). Therefore, we argue that for all samples larger than 200 identifiable charcoal pieces, the local vegetation is well represented.
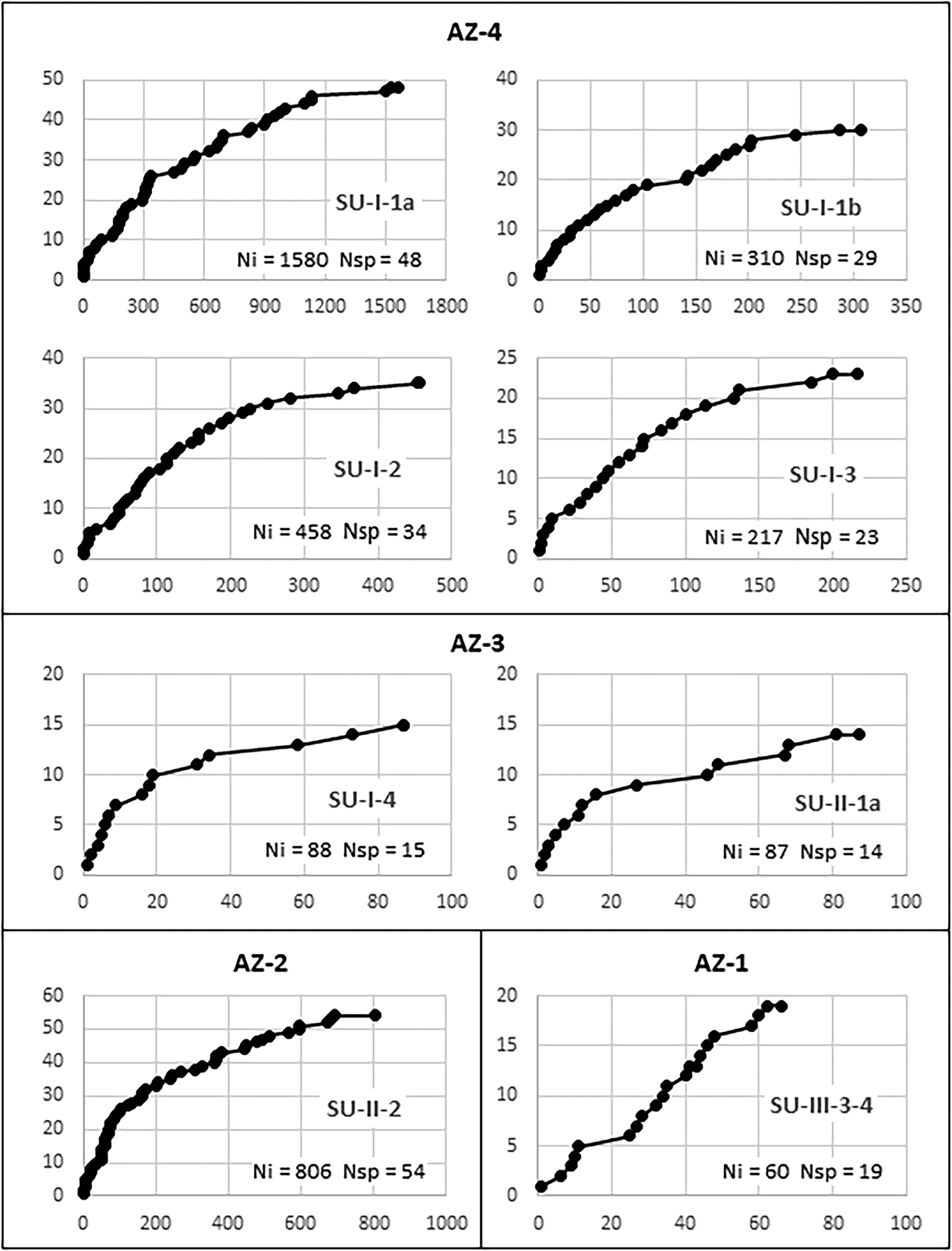
Figure 6. Saturation curves constructed for charcoal samples at different stratigraphic units of the Santa Elina rock shelter. The abscissa (x) shows the number of identified charcoal pieces; the ordinate (y) marks the first time each new species appears in the analysis. Ni = number of identified charcoal pieces; Nsp = number of taxa.
Discussion
The Santa Elina rock shelter is a place of strong symbolic value that has been the site of domestic and ritual activities for different cultural groups; it therefore holds important testimonies of past human life in Central Brazil. This site contains data on past landscapes, paleoenvironment, paleoclimate, plant use, and cultural practices, among other features, for approximately the past 30,000 years.
The present work corroborates and complements previous studies. Because of the exceptional conservation of plant remains, especially in the upper layers from the Santa Elina shelter, there have been numerous archaeobotanical analyses, notably in anthracology, carpology, palynology, fiber analysis, and plant craftwork (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Blanchot and Amenomori Reference Blanchot, Amenomori and Vilhena-Vialou2005; Ceccantini Reference Ceccantini2002; Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005; Ceccantini and Gussella Reference Ceccantini and Gussella2001; Chaves Reference Chaves and Vilhena-Vialou2005; Kamase Reference Kamase and Vilhena-Vialou2005; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005; Taveira Reference Taveira and Vilhena-Vialou2005). Nevertheless, this is the first time that researchers have analyzed plant material that encompasses the entire stratigraphic record, particularly those from the earlier Pleistocene levels.
Paleoenvironment and Landscape
Similar to previous anthracological results (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005), the taxa identified from throughout the stratigraphic record are typical of deciduous/semi-deciduous and riparian forests and the cerrado. The proportions of these plants vary over time, suggesting possible vegetation and climatic changes; yet overall, the data suggest that a wooded environment surrounded the site throughout its occupational history.
Species richness in the anthracological samples is usually less than that of modern surveys; however, with the exceptions of AZ-1 and AZ-3, in which too few samples were available for analysis, our samples were consistent with the expected values for modern flora. Analysis of between 217 and 1,580 charcoal pieces per level in AZ-2 and AZ-4 revealed 23–54 taxa from 13 to 43 families (Table 1). For comparison, modern floristic and phytosociological surveys in areas of cerrado, cerradão, and semi-deciduous forest measuring 0.5, 1, or 100 ha reported between 572 and 2,118 individuals, among which 54–68 genera were identified in 29–38 families (Arruda and Daniel Reference Arruda and Daniel2007; Camilotti et al. Reference Camilotti, Pagotto and Araujo2011; Maracahipes et al. Reference Maracahipes, Lenza, Marimon, de Oliveira, Rodrigues Pinto and Junior2011; Marimon Junior and Haridasan Reference Marimon and Haridasan2005).
Since it was first occupied, the Santa Elina rock shelter has been part of the cerrado biome. At about 27,000 cal BP (AZ-1) the site was surrounded by a forested environment. The small charcoal sample does not allow for further climatic inferences, but previous paleoecological studies corroborate the existence of a cold and humid climate in Central Brazil between about 27,000 and 20,000 BP (Salgado-Labouriau et al. Reference Salgado-Labouriau, Casseti, Ferraz-Vicentini, Martin, Soubiès, Suguio and Turcq1997). This same study demonstrated a decrease in humidity from about 18,500 BP onward, with a very dry climate until about 11,500 BP (Salgado-Labouriau et al. Reference Salgado-Labouriau, Casseti, Ferraz-Vicentini, Martin, Soubiès, Suguio and Turcq1997). During this interval, evidence of human occupation in Santa Elina shelter is scarce or nonexistent (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017).
Between about 11,000 and 10,000 cal BP (AZ-2) the occupation resumes; the site was then surrounded by a deciduous/semi-deciduous forested environment. The presence of taxa corresponding to a more open cerrado physiognomy suggests a drier climate than that of today. From about 8000 to 7000 cal BP (AZ-3), the site was still surrounded by a deciduous/semi-deciduous forest, but cerrado taxa disappear, suggesting a more humid climate than in the previous period. Nevertheless, growth rings and thick barks attest to the continued occurrence of severe dry seasons or possibly of an adaptation to fire. Indeed, the cerrado is a fire-prone biome. The open cerrado flora is typically pyrophytic; a great number of species are fire tolerant. Thick barks, typical of a high proportion of woody species in the modern flora, provide protection to trees during fire events (Coutinho Reference Coutinho1990).
Fire is an ancient and important ecological agent in the cerrado (Coutinho Reference Coutinho1990). Natural and anthropogenic fires have coexisted in its domain for thousands of years (Miranda et al. Reference Miranda, Sato, Neto, Aires and Cochrane2009): cerrado fire events have been recorded in Central Brazil since 32,400 yrs BP (Salgado-Labouriau et al. Reference Salgado-Labouriau, Casseti, Ferraz-Vicentini, Martin, Soubiès, Suguio and Turcq1997). Some authors question the human presence in this region before about 11,000 yrs BP (e.g., Schmitz Reference Schmitz1990), thus precluding the possibility of human-induced fires before the Holocene, but the chronology of the earlier occupation in Santa Elina pushes back the antiquity of human colonization.
Anthropogenic fires may be accidental or deliberate, slash-and-burn cultivation being the best-known example of the latter. Nevertheless, foragers also used fire as a tool of vegetation management to foster the growth of more palatable food plants, including secondary plants rich in carbohydrates and storage organs (Jones Reference Jones2012; Piperno and Pearsall Reference Piperno and Pearsall1998). Although there is no evidence that the inhabitants of Santa Elina may have fire-managed the cerrado, this possibility cannot be excluded. Could anthropogenic actions have been one of the drivers in the evolution of this biome? Only further studies may tell.
Finally, between about 4000 and 1500 cal BP (AZ-1), the site was surrounded by a deciduous/semi-deciduous forested environment similar to the present one and was exposed to a similar climate. There is a slight increase in the proportion of forest taxa, but no significant environmental or climatic changes can be perceived during this period. The vegetation structure seems to be the same, even if there are some changes in floristic composition.
In summary, the landscape around the shelter probably comprised, as it is today, a mosaic of vegetation types typical to the cerrado biome, consisting of patches of forest formations and of wooded, gramineous-woody, and park savannas. The anthracological results corroborate previous paleoecological studies from Central Brazil, all of which attest to colder and drier conditions and more open vegetation types during the Late Pleistocene/Early Holocene (ca. 27,000–10,000 cal BP) followed by a continuous increase in warmth, humidity, and forest expansion from around 8000 cal BP to the present (Gouveia et al. Reference Gouveia, Ruiz Pessenda, Aravena, Scheel-Ybert and Boulet2002; Ledru et al. Reference Ledru, Salgado-Labouriau and Lorscheitter1998, Reference Ledru, Ceccantini, Pessenda, Lopez, Gouveia and de Souza Ribeiro2006; Parizzi et al. Reference Parizzi, Salgado-Labouriau and Kohler1998; Salgado-Labouriau et al. Reference Salgado-Labouriau, Casseti, Ferraz-Vicentini, Martin, Soubiès, Suguio and Turcq1997). Locally, the area surrounding the shelter itself remained forested, at least during the studied periods, over the past 30,000 years. The anthracological results indicate a drier climate from the Late Pleistocene to the Early Holocene (11,000–10,000 cal BP), followed by increased humidity, associated with forest expansion and likely warmer conditions after about 8000 cal BP; by 4000 cal BP the present environmental conditions were established.
The aforementioned previous archaeobotanical analyses agree with these results, pointing to a forested environment surrounding the site and the presence of cerrado formations in the plains between 6000 and 1500 yrs BP (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Ceccantini Reference Ceccantini2002; Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005; Ceccantini and Gussella Reference Ceccantini and Gussella2001; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005). Chaves (Reference Chaves and Vilhena-Vialou2005), in a palynological analysis of seven animal coprolites dated from 4000 to 400 yrs BP, also identified a mix of forested formations and cerrado/cerradão, suggesting a vegetation similar to the present one.
Carpological analyses also agree with these results, even if the open cerrado is better represented in this case (Gussella Reference Gussella2003; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005). These analyses show that nonedible fruits/seeds account for the less frequent carpological remains found in the site and that all of them are typically forest plants (Figure 2). Conversely, edible fruits/seeds are by far the most frequent remains; all are associated with open cerrado (D. alata) formations or to associated riparian forest (H. courbaril, Humiria sp.), except for palms, which are pioneer plants and occur in forest openings. Nonedible fruits/seeds, which are dispersed by animals or wind, are probably local elements that fell from trees in the surrounding of the shelter or were brought unintentionally with branches collected for ends other than food. Food items, in contrast, were actively targeted in the plains and intentionally brought to the shelter.
In this rich and diversified landscape circulated multiple generations of hunter-gatherers (and later potters/cultivators). They ascribed meaning to the different vegetation facies according to each one's usefulness and social meaning. They created paths and probably managed some areas into durable secondary vegetation to ensure that useful plants, including palms, would thrive. Time after time they returned to Santa Elina, where they found protection and comfort.
Ancient Charcoal
In certain conditions, anthracological analysis may help discriminate sedimentary charcoal samples associated with natural fires from archaeological charcoal samples produced by human action. Sedimentary samples generally feature low taxonomic diversity because charcoal deposits occur in situ; therefore, each soil parcel will testify only to the limited number of plants that grew precisely in that spot. In contrast, dispersed archaeological charcoal samples are “synthetic” deposits, which combine remains of many firewood-gathering events, repeated over time, in the different vegetation types existing around the settlement during the occupation; therefore, they exhibit high plant diversity (Scheel-Ybert Reference Scheel-Ybert and Smith2018).
Nevertheless, a higher diversity in archaeological samples depends on long-term occupations; that is, on the occurrence of multiple firewood-gathering events (Chabal Reference Chabal1997; Scheel-Ybert Reference Scheel-Ybert and Smith2018; Théry-Parisot et al. Reference Théry-Parisot, Chabal and Chrzavzez2010). Short-term occupations produce species-poor assemblages, because they represent the remains of only few firewood-gathering/charring events (Scheel-Ybert Reference Scheel-Ybert and Smith2018).
The anthracological assemblage of the earliest occupation of Santa Elina rock shelter (SU-III), dated at about 27,000 cal BP, consists of a set of 16 samples, each one presenting between one and six taxa (Figure 5). This result is consistent with short-term human occupations, in which case temporary combustion activities would have likely produced these charcoal samples. Yet, we cannot exclude the possibility that this evidence could also have been produced by natural fires.
The archaeological context does nevertheless seem well established (Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017). These Pleistocene levels are characterized by a large amount of Glossotherium remains (cranial fragments, mandible, tooth, long bone, osteoderms, and others). Zooarchaeological analyses clearly indicate that this animal was intentionally transported to the shelter (Figuti Reference Figuti and Vilhena-Vialou2005). The bones were not in anatomical connection; the archaeological excavations revealed several scattered concentrations of small bones. The presence of modified osteoderms (polished, perforated, and beveled) and lithic and microlithic remains in association with the faunal remains testify to human activities in these Pleistocene levels.
The contemporaneity between the analyzed charcoals and Glossotherium remains is clearly established. The Glossotherium bone, dated to 27,000 ± 2000 BP (Th/U), was in clear association with three of the charcoal samples analyzed (square 26-C). One microcharcoal dated to 23,120 ± 260 BP (27,818–26,887 cal BP) was associated with four of the samples analyzed (square 27-B), reinforcing their contemporaneity (cf. Vialou et al. Reference Vialou, Benabdelhadi, Feathers, Fontugne and Vilhena-Vialou2017; Vilhena-Vialou Reference Vilhena-Vialou and Vilhena-Vialou2005).
We therefore argue that the charcoal retrieved in this unit was produced in short-term combustion features related to episodic and sporadic human activities, one of which might have involved the butchering of giant sloths.
Firewood Management and Wood Uses
During all periods of occupation of the Santa Elina rock shelter, firewood procurement was largely based on opportunistic gathering, as indicated by the high plant diversity and the good correspondence with the present phytosociology in all studied levels (cf. Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Scheel-Ybert and Solari Reference Scheel-Ybert, Solari and Vilhena-Vialou2005). Similar practices for firewood management were also demonstrated in other regional rock shelters occupations as far as 200 km south of Santa Elina, dated between 6000–200 BP (Bachelet Reference Bachelet2013, Reference Bachelet2014; Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017).
Nevertheless, some taxa might have been preferred and therefore more frequently selected for firewood; for example, Anadenanthera spp. (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017). Species of this genus are still today among the most frequent in deciduous and semi-deciduous forests (Ceccantini Reference Ceccantini and Vilhena-Vialou2005), hence their abundance is not inconsistent with the natural vegetation. Yet, their frequencies in the anthracological record are particularly high, especially in AZ-2 and AZ-4, and a cultural selection cannot be excluded. In the present, the wood of Anadenanthera species is used as firewood and for charcoal production, and the plants are also used as medicine and produce entheogenic seeds (Conceição and de Paula Reference Conceição and de Paula1990; Lorenzi Reference Lorenzi2002, Reference Lorenzi2008). Ethnobotanical inquiries in the farms around the site revealed that Anadenanthera colubrina and A. macrocarpa are by far the two most preferred species for firewood cooking among contemporary farmers (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017; Scheel-Ybert Reference Scheel-Ybert1997).
Cultural selection of bamboos (Guadua sp. and possibly another species) is also suggested, especially in the more recent occupations. The high frequency of bamboo stems in the shelter, carbonized or not, points to the large use of this material. Bamboos were frequently used as posts (Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005); they may also have been used to produce different utilitarian artifacts. But it is the high frequency of charred remains that stands out. Bamboo pieces are among the most important charred remains at AZ-4 (ca. 4000–1500 cal BP). No evidence of this material was retrieved in AZ-3 (ca. 8000–7000 cal BP), but a few fragments were identified in AZ-2 (ca. 11,000–10,000 cal BP). Today, bamboo is considered a poor-quality firewood because it is fast burning and inclined to bursts. Nevertheless, the great quantity of charred bamboo pieces in dispersed and concentrated charcoal indicates their charring was not accidental. Bachelet and Scheel-Ybert (Reference Bachelet and Scheel-Ybert2017) suggested that it might have been used as kindling and therefore specifically collected for this purpose, but it is also possible that bamboos were mostly collected for other utilitarian uses (such as posts, fences, and other) and that the remains of these activities were secondarily used as kindling.
In addition to the many wood varieties notably used for posts (Ceccantini and Fernandez Reference Ceccantini, Fernandez and Vilhena-Vialou2005), wood probably was used by these communities for many purposes that unfortunately are not preserved in the archaeological record, such as a wide range of artifacts, handles, construction material, medicines, and others.
Conclusion
The Santa Elina rock shelter was a good place to live. In addition to the attractiveness of a rock shelter, the permanent forest made this site a sort of vegetation refuge, a protected and pleasant site where people might find shelter from bad weather and from cold and hot days, while having access to particularly rich environments. The open cerrado in the plains around the site was largely exploited for food procurement and useful plants, whereas the deciduous/semi-deciduous forest around the shelter provided wood, firewood, and protection from seasonal warmth and aridity.
In all periods of the occupation of this shelter, human groups adopted opportunistic firewood-gathering strategies, probably collecting the deadwood available in the vegetation surrounding the shelter. Opportunistic gathering was probably coincident with cultural selection of some plants, especially Anadenanthera (angico), which might have been praised and selected as a good firewood in this region for millennia, as suggested by the combination of anthracological and ethnographic results. Bamboos as well were probably subject to cultural selection, especially during the late Holocene, when they were likely used as kindling, as well as posts and material for craftwork (Bachelet and Scheel-Ybert Reference Bachelet and Scheel-Ybert2017).
The earliest human occupations of Santa Elina rock shelter, during the Late Pleistocene, were probably sporadic and of short duration, as suggested by the extremely low diversity of charcoal deposits. The transition between the Pleistocene/Holocene exhibits an increase in the frequency and intensity of occupation, attested by the high charcoal diversity. It culminates with a very intense occupation of the shelter during the Late Holocene, as indicated not only by the high charcoal diversity but also by the huge quantities of charcoal and plant remains preserved in the upper layers of this site.
This emblematic site of Central Brazil, of rare beauty but also deeply symbolic, is key to our understanding of the first routes of colonization in South America. Most of all, it bears important information on the ways of life of Central Brazilian hunter-gatherers, some of which we have tried to present in this article.
Acknowledgments
Although the first anthracological microscopic analyses that are discussed in this paper were performed at the DBAE, in Montpellier, France (Equipe Dynamiques de la Biodiversité et Anthropo-Ecologie—ISEM UMR 5554, Institut des Sciences de l'Evolution de Montpellier), most of the work here presented was developed in the Museu Nacional in Rio de Janeiro. Many charcoal samples from this site were stored at the Laboratory of Archaeobotany and Landscape of the Museu Nacional, where most of this research took place. Unfortunately, all this material was destroyed during the catastrophic fire in September 2018. This terrible loss is irreparable and insurmountable. Fortunately, the analysis had been completed, the data are preserved, and we are now able to disclose the results to the academic community. We thank the staff of the Museu Nacional/Federal University of Rio de Janeiro, Museu de Arqueologia e Etnologia of the University of São Paulo, National Museum of Natural History in Paris, DBAE, and Ministère des Affaires Etrangères et du Développement International in Paris, for supporting different stages of this research. We also thank the Foundation for the Coordination and Improvement of Higher Level or Education Personnel (CAPES) for the fellowship that made part of this research possible. R. Scheel-Ybert is a CNPq (National Counsel of Technological and Scientific Development) and FAPERJ (Carlos Chagas Filho Research Support Foundation) fellowship holder. We acknowledge Ana Paula Alcaraz and Luke Stroth for their careful revisions and Agueda Vilhena-Vialou, Denis Vialou, Levy Figuti, and two anonymous reviewers for comments and suggestions that much improved the manuscript.
Data Availability Statement
The data supporting this study are available from the corresponding author upon request.
Supplemental Materials
For supplementary material accompanying this article, visit https://doi.org/10.1017/laq.2020.3.
Supplemental Figure 1. Full-size anthracological diagram for the Santa Elina rock shelter.









