Published online by Cambridge University Press: 08 April 2021
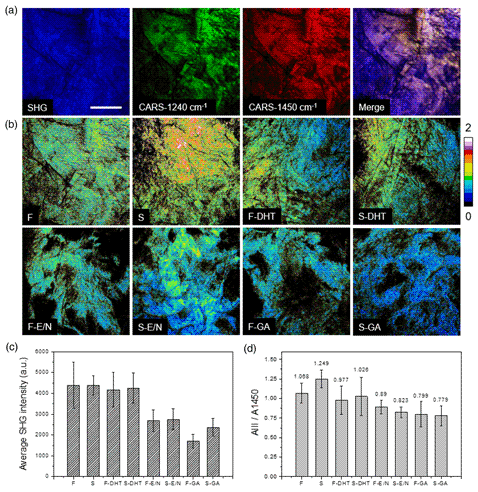
Engineered biomaterials provide unique functions to overcome the bottlenecks seen in biomedicine. Hence, a technique for rapid and routine tests of collagen is required, in which the test items commonly include molecular weight, crosslinking degree, purity, and sterilization induced structural change. Among them, the crosslinking degree mainly influences collagen properties. In this study, second harmonic generation (SHG) and coherent anti-Stokes Raman scattering (CARS) microscopy are used in combination to explore the collagen structure at molecular and macromolecular scales. These measured parameters are applied for the classification and quantification among the different collagen scaffolds, which were verified by other conventional methods. It is demonstrated that the crosslinking status can be analyzed from SHG images and presented as the coherency of collagen organization that is correlated with the mechanical properties. Also, the comparative analyses of SHG signal and relative CARS signal of amide III band at 1,240 cm−1 to δCH2 band at 1,450 cm−1 of these samples provide information regarding the variation of the molecular structure during a crosslinking process, thus serving as nonlinear optical signatures to indicate a successful crosslinking.
Chao-Wei Hung and Nirmal Mazumder equally contributed to the present study.