Published online by Cambridge University Press: 20 May 2022
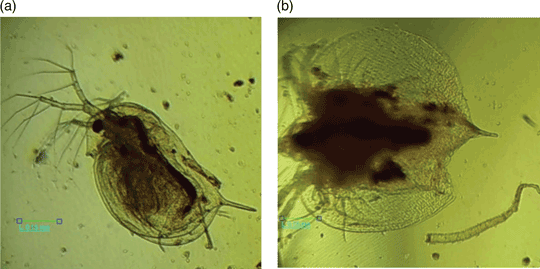
Five compounds 2-(2-hydroxybenzylidene)-N-(prop-2-en-1-yl)hydrazinecarbothioamide (H2L), bis[μ2-2-({2-[(prop-2-en-1-yl)carbamothioyl]hydrazinylidene}methyl)phenolato-S,N,O:O]diaquadicopper(II) nitrate (1), bis[μ2-2-({2-[(prop-2-en-1-yl)carbamothioyl]hydrazinylidene}methyl)phenolato-S,N,O:O]diimidazoldicopper(II) nitrate (2), bis[μ2-2-({2-[(prop-2-en-1-yl)carbamothioyl]-hydrazinylidene}methyl)phenolato-S,N,O:O]bis-(3,5-dibromopyridine)dicopper(II) nitrate (3), bis[μ2-2-({2-[(prop-2-en-1-yl)carbamothioyl]-hydrazinylidene}methyl)phenolato-S,N,O:O]bis(4-methylpyridine)dicopper(II) nitrate hexahydrate (4) were synthesized. The antiproliferative properties of these compounds toward cancer cell lines RD, HeLa, and normal cell line MDCK have been investigated. The tested complexes surpass Doxorubicin (DOXO) in the efficiency of anticancer activity as their IC50 values toward cancer cells are lower than the corresponding values of DOXO and the selectivity indexes exceed the corresponding SI value of DOXO. The tested compounds demonstrated a high antioxidant effect against ABTS•+ radical cations as well as low toxicity on Daphnia magna.