Published online by Cambridge University Press: 03 September 2020
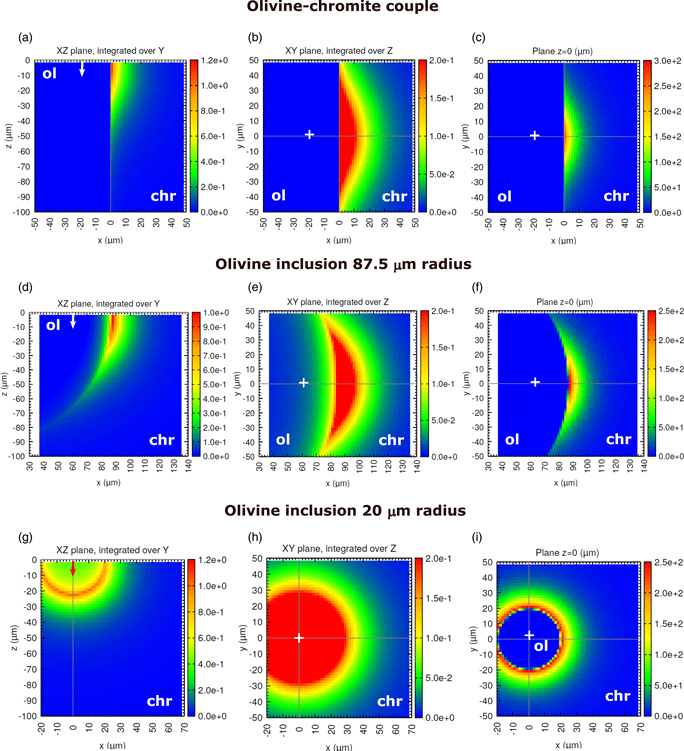
One of the limiting factors for the analysis of minor elements in multiphase materials by electron probe microanalysis is the effect of secondary fluorescence (SF), which is not accounted for by matrix corrections. Although the apparent concentration due to SF can be calculated numerically or measured experimentally, detailed investigations of this effect for fine-grained materials are scarce. In this work, we use the Monte Carlo simulation program PENEPMA to examine and correct the effect of SF affecting micron-sized mineral inclusions hosted by other minerals. A concentration profile across an olivine [(Mg,Fe)2SiO4] inclusion in chromite (Fe2+Cr2O4) is measured and used to assess the reliability of calculations, where different boundary geometries are examined. Three application examples are presented, which include the determination of Cr in olivine and serpentine [Mg3Si2O5(OH)4] inclusions hosted by chromite and of Fe in quartz (SiO2) inclusions hosted by almandine garnet (Fe3Al2Si3O12). Our results show that neglecting SF leads to concentrations that are overestimated by ~0.1–0.8 wt%, depending on inclusion size. In addition, assuming a straight boundary yields to an underestimation of SF effects by a factor of ~2–4. Because its long-range nature, SF severely compromises trace element analyses even for phases as large as 1 mm in size.