Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Meier, Martin S
Jones, Megan E
Felfer, Peter J
Bagot, Paul A J
Moody, Michael P
and
Haley, Daniel
2022.
Exploiting Adsorption Dynamics in Atom Probe Tomography for accurate Measurements of Hydrogen Concentrations.
Microscopy and Microanalysis,
Vol. 28,
Issue. S1,
p.
1650.
Tehranchi, Ali
Chakraborty, Poulami
López Freixes, Martí
McEniry, Eunan J.
Gault, Baptiste
Hickel, Tilmann
and
Neugebauer, Jörg
2023.
Tailoring negative pressure by crystal defects: Microcrack induced hydride formation in Al alloys.
Physical Review Materials,
Vol. 7,
Issue. 10,
Zhou, Ziyang
Wang, Zhengquan
Niu, Ranming
Liu, Pang-Yu
Huang, Chao
Sun, Yi-Hsuan
Wang, Xiutong
Yen, Hung-Wei
Cairney, Julie M.
and
Chen, Yi-Sheng
2023.
Cryogenic atom probe tomography and its applications: a review.
Microstructures,
Vol. 3,
Issue. 4,
Mayweg, David
Eriksson, Johan
Bäcke, Olof
Breen, Andrew J.
and
Thuvander, Mattias
2023.
Focused Ion Beam induced hydride formation does not affect Fe, Ni, Cr-clusters in irradiated Zircaloy-2.
Journal of Nuclear Materials,
Vol. 581,
Issue. ,
p.
154444.
Gault, Baptiste
Saksena, Aparna
Sauvage, Xavier
Bagot, Paul
Aota, Leonardo S
Arlt, Jonas
Belkacemi, Lisa T
Boll, Torben
Chen, Yi-Sheng
Daly, Luke
Djukic, Milos B
Douglas, James O
Duarte, Maria J
Felfer, Peter J
Forbes, Richard G
Fu, Jing
Gardner, Hazel M
Gemma, Ryota
Gerstl, Stephan S A
Gong, Yilun
Hachet, Guillaume
Jakob, Severin
Jenkins, Benjamin M
Jones, Megan E
Khanchandani, Heena
Kontis, Paraskevas
Krämer, Mathias
Kühbach, Markus
Marceau, Ross K W
Mayweg, David
Moore, Katie L
Nallathambi, Varatharaja
Ott, Benedict C
Poplawsky, Jonathan D
Prosa, Ty
Pundt, Astrid
Saha, Mainak
Schwarz, Tim M
Shang, Yuanyuan
Shen, Xiao
Vrellou, Maria
Yu, Yuan
Zhao, Yujun
Zhao, Huan
and
Zou, Bowen
2024.
Towards Establishing Best Practice in the Analysis of Hydrogen and Deuterium by Atom Probe Tomography.
Microscopy and Microanalysis,
Tunes, Matheus A.
Uggowitzer, Peter J.
Dumitraschkewitz, Phillip
Willenshofer, Patrick
Samberger, Sebastian
da Silva, Felipe C.
Schön, Cláudio G.
Kremmer, Thomas M.
Antrekowitsch, Helmut
Djukic, Milos B.
and
Pogatscher, Stefan
2024.
Limitations of Hydrogen Detection After 150 Years of Research on Hydrogen Embrittlement.
Advanced Engineering Materials,
Jakob, Severin
Sattari, Mohammad
Sefer, Birhan
Ooi, Steve
and
Thuvander, Mattias
2024.
Characterization of hydrogen traps in a co-precipitation steel investigated by atom probe experiments without cryogenic transfer.
Scripta Materialia,
Vol. 243,
Issue. ,
p.
115963.
Tegg, Levi
McCarroll, Ingrid E
Kim, Se-Ho
Dubosq, Renelle
Woods, Eric V
El-Zoka, Ayman A
Gault, Baptiste
and
Cairney, Julie M
2024.
Analysis of Water Ice in Nanoporous Copper Needles Using Cryo Atom Probe Tomography.
Microscopy and Microanalysis,
Mayweg, David
Eriksson, Johan
Sattari, Mohammad
and
Thuvander, Mattias
2024.
Limits of hydrogen analysis by atom probe tomography targeting Zr(Fe,Cr)2 second phase particles in Zr-based fuel cladding from reactor operation.
Journal of Nuclear Materials,
Vol. 601,
Issue. ,
p.
155343.
Ott, Benedict
Heller, Martina
Monajem, Mehrpad
and
Felfer, Peter
2024.
Miniaturized gas exposure devices for atom probe experiments.
Microscopy Research and Technique,
Vol. 87,
Issue. 9,
p.
2113.
Saksena, Aparna
Sun, Binhan
Dong, Xizhen
Khanchandani, Heena
Ponge, Dirk
and
Gault, Baptiste
2024.
Optimizing site-specific specimen preparation for atom probe tomography by using hydrogen for visualizing radiation-induced damage.
International Journal of Hydrogen Energy,
Vol. 50,
Issue. ,
p.
165.
 ${\rm H}_2^ +$) originating from the measurement environment can overlap with deuterium (D+) in the mass-to-charge-state spectrum, thus preventing the direct application of isotopic marking for unambiguous hydrogen analysis. First, we applied an existing method for hydrogen content estimation, using
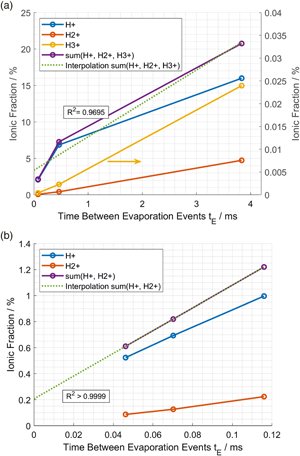
${\rm H}_2^ +$) originating from the measurement environment can overlap with deuterium (D+) in the mass-to-charge-state spectrum, thus preventing the direct application of isotopic marking for unambiguous hydrogen analysis. First, we applied an existing method for hydrogen content estimation, using  ${\rm H}^ + {/}{\rm H}_2^ +$ ratios obtained from paired deuterated/nondeuterated experiments. These measurements demonstrated sufficient residual uncertainty to motivate exploring an alternative method to accurately estimate hydrogen content. By varying the time between evaporation events, it is then shown that a highly correlated relationship between field evaporation rate and hydrogen content exists and can also be used to predict hydrogen content. This leads to a new method for measuring hydrogen content within the specimen. We combine this extrapolation technique with continuous cycling of the evaporation rate or pulse frequency during an APT experiment. This could enable spatially resolved imaging of hydrogen concentrations despite the presence of a contaminant background hydrogen signal, without the need for deuteration.
${\rm H}^ + {/}{\rm H}_2^ +$ ratios obtained from paired deuterated/nondeuterated experiments. These measurements demonstrated sufficient residual uncertainty to motivate exploring an alternative method to accurately estimate hydrogen content. By varying the time between evaporation events, it is then shown that a highly correlated relationship between field evaporation rate and hydrogen content exists and can also be used to predict hydrogen content. This leads to a new method for measuring hydrogen content within the specimen. We combine this extrapolation technique with continuous cycling of the evaporation rate or pulse frequency during an APT experiment. This could enable spatially resolved imaging of hydrogen concentrations despite the presence of a contaminant background hydrogen signal, without the need for deuteration.