Published online by Cambridge University Press: 30 October 2020
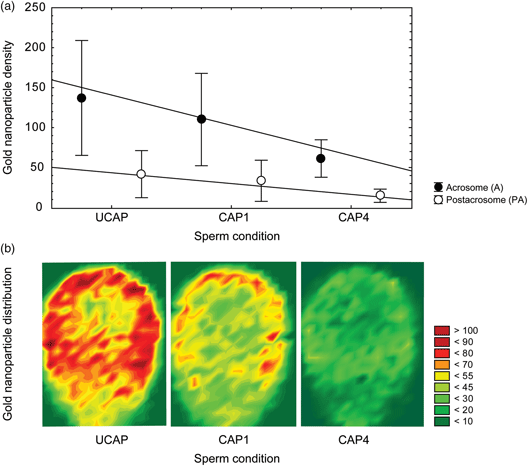
Sperm capacitation includes the reorganization of plasma membrane components and the outstanding modification of the glycocalyx. The α-mannose presence and location during in vitro capacitation have been commonly described in human spermatozoa using Concanavalin A (Con A) lectin. However, it is still unclear to date how in vitro capacitation time affects the α-mannose residues and their topographic spatial distribution on sperm membrane. Here, we characterized the α-mannose density and specific membrane domain locations before and after in vitro capacitation (1–4 h) using high-resolution field emission scanning electron microscopy (FE-SEM). Results showed that α-mannose residues were present preferably on the acrosome domains for all physiological conditions. Uncapacitated sperm comparatively exhibits significant highest labeling densities of α-mannose residues. In addition, as in vitro capacitation takes place, significant and progressive decreasing of sugar residues was combined with their relocation mostly affecting acrosomal domain apical areas. Our findings reveal that combined approach using FE-SEM and gold nanoparticle topographical mapping offers new human sperm biomolecular and structural details during capacitation events.
M. J. Gómez-Torres and L. Robles-Gómez contributed equally as co-first authors.