Published online by Cambridge University Press: 04 July 2022
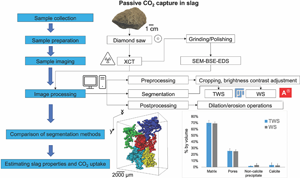
Weathering of silicate-rich industrial wastes such as slag can reduce emissions from the steelmaking industry. During slag weathering, different minerals spontaneously react with atmospheric CO2 to produce calcite. Here, we evaluate the CO2 uptake during slag weathering using image-based analysis. The analysis was applied to an X-ray computed tomography (XCT) dataset of a slag sample associated with the former Ravenscraig steelworks in Lanarkshire, Scotland. The element distribution of the sample was studied using scanning electron microscopy (SEM), coupled with energy-dispersive spectroscopy (EDS). Two advanced image segmentation methods, namely trainable WEKA segmentation in the Fiji distribution of ImageJ and watershed segmentation in Avizo ® 9.3.0, were used to segment the XCT images into matrix, pore space, calcite, and other precipitates. Both methods yielded similar volume fractions of the segmented classes. However, WEKA segmentation performed better in segmenting smaller pores, while watershed segmentation was superior in overcoming the partial volume effect presented in the XCT data. We estimate that CO2 has been captured in the studied sample with an uptake between 20 and 17 kg CO2/1,000 kg slag for TWS and WS, respectively, through calcite precipitation.