Published online by Cambridge University Press: 05 September 2022
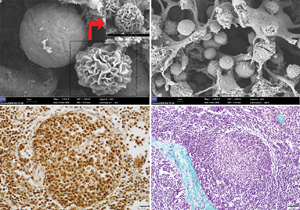
Toll-like receptor (TLR)-mediated inflammatory processes play a critical role in the innate immune response during the initial interaction between the infecting microorganism and immune cells. This study aimed to investigate the possible microanatomical and histological differences in mandibular and bronchial lymph nodes in Akkaraman and Romanov lambs induced by lipopolysaccharide (LPS) and lipoteichoic acid (LTA) and study the gene, protein, and immunoexpression levels of TLR4, myeloid differentiation factor 88 (MyD88), and tumor necrosis factor-α (TNF-α) that are involved in the immune system. Microanatomical examinations demonstrated more intense lymphocyte infiltration in the bronchial lymph nodes of Akkaraman lambs in the LPS and LTA groups compared to Romanov lambs. TLR4, MyD88, and TNF-α immunoreactivities were more intense in the experimental groups of both breeds. Expression levels of MyD88 and TNF-α genes in the bronchial lymph node of Akkaraman lambs were found to increase statistically significantly in the LTA group. TLR4 gene expression level in the mandibular lymph node was found to be statistically significantly higher in the LTA + LPS group. In conclusion, dynamic changes in the immune cell populations involved in response to antigens such as LTA and LPS in the lymph nodes of both breeds can be associated with the difference in the expression level of the TLR4/MyD88/TNF-α genes.