Published online by Cambridge University Press: 01 December 2021
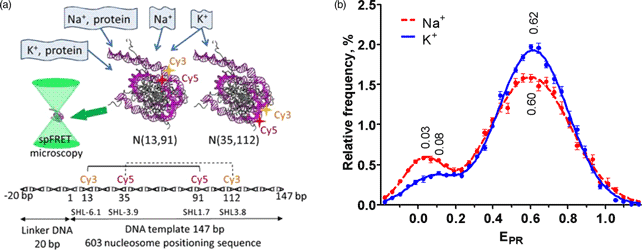
Inorganic ions are essential factors stabilizing nucleosome structure; however, many aspects of their effects on DNA transactions in chromatin remain unknown. Here, differential effects of K+ and Na+ on the nucleosome structure, stability, and interactions with protein complex FACT (FAcilitates Chromatin Transcription), poly(ADP-ribose) polymerase 1, and RNA polymerase II were studied using primarily single-particle Förster resonance energy transfer microscopy. The maximal stabilizing effect of K+ on a nucleosome structure was observed at ca. 80–150 mM, and it decreased slightly at 40 mM and considerably at >300 mM. The stabilizing effect of Na+ is noticeably lower than that of K+ and progressively decreases at ion concentrations higher than 40 mM. At 150 mM, Na+ ions support more efficient reorganization of nucleosome structure by poly(ADP-ribose) polymerase 1 and ATP-independent uncoiling of nucleosomal DNA by FACT as compared with K+ ions. In contrast, transcription through a nucleosome is nearly insensitive to K+ or Na+ environment. Taken together, the data indicate that K+ environment is more preserving for chromatin structure during various nucleosome transactions than Na+ environment.