Published online by Cambridge University Press: 17 March 2022
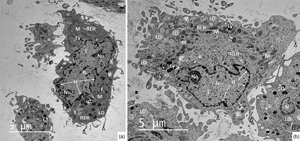
The present study was designed to compare the ultrastructure of early endothelial progenitor cells (EPCs) derived from rabbit peripheral blood (PB-EPCs) and bone marrow (BM-EPCs). After the cells had been isolated and cultivated up to passage 3, microphotographs obtained from transmission electron microscope were evaluated from qualitative and quantitative (unbiased stereological approaches) points of view. Our results revealed that both cell populations displayed almost identical ultrastructural characteristics represented by abundant cellular organelles dispersed in the cytoplasm. Moreover, the presence of very occasionally occurring mature endothelial-specific Weibel–Palade bodies (WPBs) confirmed their endothelial lineage origin. The more advanced stage of their differentiation was also demonstrated by the relatively low nucleus/cytoplasm (N/C) ratios (0.41 ± 0.19 in PB-EPCs; 0.37 ± 0.25 in BM-EPCs). Between PB-EPCs and BM-EPCs, no differences in proportions of cells occupied by nucleus (28.13 ± 8.97 versus 25.10 ± 11.48%), mitochondria (3.71 ± 1.33 versus 4.23 ± 1.00%), and lipid droplets (0.65 ± 1.01 versus 0.36 ± 0.40%), as well as in estimations of the organelles surface densities were found. The data provide the first quantitative evaluation of the organelles of interest in PB-EPCs and BM-EPCs, and they can serve as a research framework for understanding cellular function.