Article contents
Structural and Ultrastructural Analysis of the Multiple Myeloma Cell Niche and a Patient-Specific Model of Plasma Cell Dysfunction
Published online by Cambridge University Press: 09 December 2021
Abstract
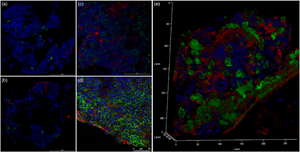
Multiple myeloma (MM) is a deadly, incurable malignancy in which antibody-secreting plasma cells (PCs) become neoplastic. Previous studies have shown that the PC niche plays a role cancer progression. Bone marrow (BM) cores from MM and a premalignant condition known as monoclonal gammopathy of unknown significance (MGUS) patients were analyzed with confocal and transmission electron microscopy. The BM aspirates from these patients were used to generate 3D PC cultures. These in vitro cultures were then assayed for the molecular, cellular, and ultrastructural hallmarks of dysfunctional PC at days 1 and 5. In vivo, evidence of PC endoplasmic reticulum stress was found in both MM and MGUS BM; however, evidence of PC autophagy was found only in MM BM. Analysis of in vitro cultures found that MM PC can survive and maintain a differentiated phenotype over an unprecedented 5 days, had higher levels of paraprotein production when compared to MGUS-derived cultures, and showed evidence of PC autophagy as well. Increased fibronectin deposition around PC associated with disease severity and autophagy dysregulation was also observed. 3D cultures constructed from BM aspirates from MGUS and MM patients allow for long-term culture of functional PC while maintaining their distinct morphological phenotypes.
Keywords
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
Footnotes
These two authors are co-senior authors.
References
- 5
- Cited by



