Introduction
Biomineralized, hard eggshells are known from reptiles and birds throughout the geological record. These eggshells represent palaeoenvironmental recorders and biomarkers for stratigraphy (Pickford & Senut, Reference Pickford and Senut1999; Senut, Reference Senut2000) and have been used for phylogeny (Erben, Reference Erben1970; Adeyeye, Reference Adeyeye2009; Pickford, Reference Pickford2014). Also, avian eggs are a food resource for humans and numerous animals and are used in cosmetics, dentistry, reconstructive surgery, and for vaccines, among other uses (Dupoirieux, Reference Dupoirieux1999; Dupoirieux et al., Reference Dupoirieux, Pourquier, Neves and Téot2001; Uygur et al., Reference Uygur, Ozmen, Kandal, Lortlar, Omeroglu, Arac and Cenetoglu2011; Kattimani et al., Reference Kattimani, Chakravarthi, Kanumuru, Subbarao, Sidharthan, Sampath Kumar and Prasad2014). These studies of eggshell structure and composition were mainly based on domesticated chicken eggs.
Nathusius (Reference Nathusius von Königsborn1868, Reference Nathusius von Königsborn1893) described the structure of avian eggshells of various species. From sections of the thick ostrich eggshells (>2 mm), the main structural layers were identified as follows: inner organic membranes, the mammillary layer, the thick spongy layer, and the thin outer prismatic layer, as well as the complex branched pores. In these large and thick eggshells, curved growth lines are well visible. The main part of the eggshell was described as composed of CaCO3, rich in Mg and P, and with minor concentrations of Fe and S, still there was no mention of the CaCO3 polymorph (calcite and/or aragonite). The main layers described by Nathusius in chicken and ostrich eggshells were largely adopted, but there is no agreement on the descriptive terms (Romanov & Romanov, Reference Romanov and Romanov1949; Erben, Reference Erben1970; Becking, Reference Becking1975; Hincke et al., Reference Hincke, Nys, Gautron, Mann, Rodriguez-Navarro and McKee2012). One of the first microstructural and mineralogical analyses of modern and fossil eggshells of reptiles and birds using scanning electron microscopy (SEM) and X-ray diffraction was conducted by Erben (Reference Erben1970). All bird and reptile eggshells are calcite, except for Testudines (turtles), called Chelonia in Erben (Reference Erben1970), in which aragonite was the mineral component (Fig. 1). Then, Solomon & Baird (Reference Solomon and Baird1976) and Baird & Solomon (Reference Baird and Solomon1979), using X-ray diffraction and infrared analyses, found calcite and aragonite in the inner part of the eggshell of Chelonia mydas and noticed that such bi-mineralic structure is known only in farm-reared samples.
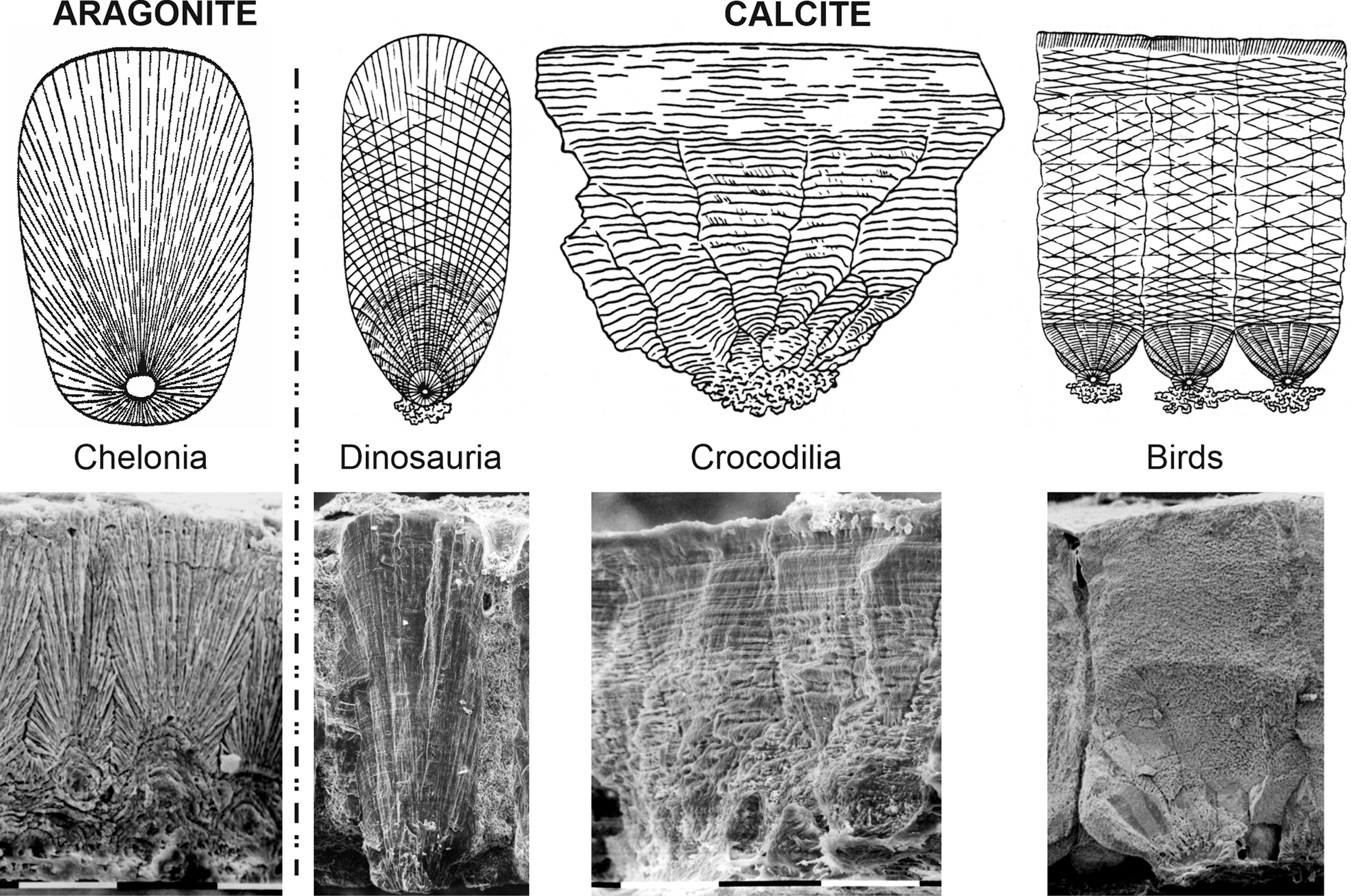
Fig. 1. Structural categories of calcified modern and fossil eggshells of reptiles and birds. Drawings from Erben (Reference Erben1970).
Numerous papers are dedicated to the structure and composition of the avian eggshells (Heyn, Reference Heyn1963; Cain & Heyn, Reference Cain and Heyn1964; Tyler, Reference Tyler1969a, Reference Tyler1969b; Becking, Reference Becking1975). Tullett et al. (Reference Tullett, Board, Love, Perrott and Scott1976) have found a compositional difference in the outer layer of sea-bird eggshells: the organic cuticle usually described in “terrestrial” birds is replaced by a vateritic layer. Vaterite was also detected at the surface of the eggs of Crotophaga major (Portugal et al., Reference Portugal, Bowen and Riehk2018) and in nonparasitic cuckoos (Board & Perrott, Reference Board and Perrott1979). However, the main part of these eggshells was calcite. Lastly, the eggshells of an American passerine, Setophaga ruticilla (Linnaeus, 1758), were described as composed of “aragonite having a massive structure with aeration holes on the inner portion of the shell with a thin layer of microcrystalline aragonite as a coating” (Kyser et al., Reference Kyser, Radcliffe and Langin2007). The description of the presence of aragonite, as the main mineral constituent, of passerine eggshells is in conflict with the use of both the structure and mineralogy for the “phylogeny” of reptile and bird eggshells (Erben, Reference Erben1970; Supplementary Fig. S1). According to this concept, the first stage of differentiation was between aragonitic eggshells of Chelonia and calcitic eggshells; the second stage was between birds and “calcitic” reptiles and birds. Up to now, the oldest known bird eggshell (Lower Cretaceous of Japan) is calcite and shows a three-layered structure (Imai & Azuma, Reference Imai and Azuma2014).
Setophaga (Swainson, 1827) is a genus of the Parulidae family, also called New World warblers. Twenty-five genera were traditionally assigned to this family (American Ornithologists’ Union, 1998). The monophyly of Parulidae and the phylogenetic boundaries of the family were controversial. Studies using molecular analyses have shown that some traditional families of Passerida are not monophyletic (Klicka et al., Reference Klicka, Johnson and Lanyon2000). Nevertheless, mitochondrial and nuclear DNA sequences have shown that Setophaga is one of the 19 genera of the Parulidae (Lovette & Bermingham, Reference Lovette and Bermingham2002). So, the aragonitic composition of the eggshell of Setophaga would disagree with this monophyly.
The aim of this study is to present a structural and compositional analysis of passerine eggshells to revisit the findings by Kyser et al. (Reference Kyser, Radcliffe and Langin2007). If the aragonite composition is confirmed, the eggshell phylogeny would have to be revised. However, Erben's phylogeny (Reference Erben1970) would prevail if these eggshells are calcitic. So, we try to answer some questions: What is the structure of the eggshell of Setophaga? What about its mineralogy? If these eggshells are aragonitic, where they are in the “phylogeny” proposed by Erben (Reference Erben1970) (Supplementary Fig. S1).
Materials and Methods
Material (Fig. 2, Supplementary Fig. S2)
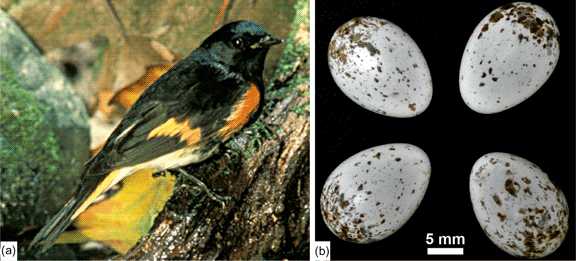
The American redstart, Setophaga ruticilla (Linnaeus, 1758), is a small bird (11–14 cm long with the tail) that is migratory between North America and the north of South America (Supplementary Fig. S2). They are in North America for the breeding season. Sexual dimorphism is variable: the male has black and orange feathers, whereas the colors of the female are not so bright (mainly yellow, pale green, and gray). The female lays between two and five white or cream-colored eggs speckled with varying amounts of brown (Fig. 2). The average size of eggs is 17 × 12 mm.

Fig. 2. Setophaga and its eggs. (a) Adult male with vivid colors (https://fr.wikipedia.org/wiki/Paruline_flamboyante#/media/Fichier:Setophaga_ruticilla.jpg). Fish and wildlife service public domain. (b) Outer views of eggs showing the irregular brown spots, collected between 7 May and 30 June 1900 (see original labels in Supplementary Fig. S2); collections of the western foundation of vertebrate zoology.
Small fragments of eggshells obtained from the collections of the Western Foundation of Vertebrate Zoology. Fragmentary eggshell materials, collected in Newfoundland (Canada), Michigan, and Connecticut (USA), were used in this study.
Scanning Electron Microscopy
Gold-coated fractures, as well as inner and outer surfaces of the eggshells, were seen using a Zeiss EVO MA10 (Institut de Physique du Globe de Paris) and a Zeiss Gemini LEO 1550 (Max Planck Institute of Colloids and Interfaces, Potsdam) in secondary electron mode. Uncoated samples were examined using an FEI QUANTA FEG 600 in low vacuum and both back scattered electron (BSE) and secondary electron (SE) modes (Max Planck Institute of Colloids and Interfaces, Potsdam).
BSE consists of high-energy electrons that are reflected or back-scattered out of the specimen. Since atoms with a high atomic number Z are stronger scattered than light ones, they appear brighter and the resulting images contain compositional information. CaCO3 biominerals contain Mg, P, and Sr (Romanov & Romanov, Reference Romanov and Romanov1949; Jenkins, Reference Jenkins1975; Dauphin, Reference Dauphin1988; Dalbeck & Cusack, Reference Dalbeck and Cusack2006). These elements have higher atomic numbers than the organic components (mainly H, N, C, and O). As a result, mineral-enriched zones are brighter than those enriched in organic components.
Tomography
For the tomographic analyses, the high-resolution nanofocus computer tomography system “X-Ray Micro CT EasyTom 160” device by the company RX Solutions was used. This device includes two X-ray tubes, each with three different focal spot modes. The microtube is equipped with a tungsten filament and provides maximum power of 150 kV and 500 μA. It can reach voxel sizes between 89.00 and 4.00 μm. The nanotube is equipped with a LaB6 filament and provides maximum power of 100 kV and 200 μA. It can reach voxel sizes between 4.00 and 0.40 μm. In addition, a caesium iodide flat panel detector is installed.
The scan was performed by using the nanotube with 80 kV and 80 μA at the small focal spot mode. The source–detector distance was 793.31 mm, the source–object distance was 2.50 mm and the resulting voxel size was 0.40 μm. The frame rate of the flat panel detector was 0.25 with an average of 10 frames. By collecting 1,120 images per turn, the scan time was 15 h after a calibration period of 2 h. 3D reconstructions were done using FIJI and Amira software.
Fourier Transform Infrared Spectrometry
Fourier transform infrared spectrometry (FTIR) absorption spectrometry was carried out at the Centre de recherche sur la Conservation, CRC USR 3224 Paris) using a Continuum IR microscope coupled to a Nexus FTIR bench (Thermo Nicolet). The microscope was operated in reflection mode, where the Schwarzschild objective has a magnification of 32. Spectra were collected using a Nicolet with a resolution of 4 cm−1 and an aperture of 75 × 75 μm. For each spectrum, 200 scans were accumulated in the wavenumber range 4,000–700 cm−1.
FTIR spectra of the calcite and aragonite groups are characterized by three major bands attributed to the carbonate ion ![]() ${{\rm CO}_3}^{2-}$ : ν3 at 1,429 cm−1, the ν2 doublet 877–848 cm−1, and ν4 at 713 cm−1 for calcite; ν1 at 1,471 cm−1, two doublets ν2 at 858–844 cm−1, and ν4 at 713–700 cm−1 for aragonite (Adler & Kerr, Reference Adler and Kerr1962). Bands in these doublets are of unequal intensities. The weak carbonate ν1 band is at 1,012 cm−1 for calcite and at 1,083 cm−1 for aragonite. According to Ylmen & Jäglid (Reference Ylmen and Jäglid2013), the ν3 asymmetric stretching of
${{\rm CO}_3}^{2-}$ : ν3 at 1,429 cm−1, the ν2 doublet 877–848 cm−1, and ν4 at 713 cm−1 for calcite; ν1 at 1,471 cm−1, two doublets ν2 at 858–844 cm−1, and ν4 at 713–700 cm−1 for aragonite (Adler & Kerr, Reference Adler and Kerr1962). Bands in these doublets are of unequal intensities. The weak carbonate ν1 band is at 1,012 cm−1 for calcite and at 1,083 cm−1 for aragonite. According to Ylmen & Jäglid (Reference Ylmen and Jäglid2013), the ν3 asymmetric stretching of ![]() ${{\rm CO}_3}^{2-}$ has a larger wavenumber range from 1,425 and 1,590 cm−1, so that an overlap with amide II bands exists.
${{\rm CO}_3}^{2-}$ has a larger wavenumber range from 1,425 and 1,590 cm−1, so that an overlap with amide II bands exists.
For vaterite, ν1 band is at 1,085–1,090 cm−1, ν2 band is a doublet at 877–850 cm−1 (Jones & Jackson, Reference Jones and Jackson1993), or 878–870 cm−1 (Andersen & Brecevic, Reference Andersen and Brecevic1991); ν3 is a doublet (1,491–1,420 cm−1 or 1,487–1,445 cm−1) (Andersen & Brecevic, Reference Andersen and Brecevic1991), or a triplet 1,408–1,432–1,489 cm−1 (Jones & Jackson, Reference Jones and Jackson1993); ν4 doublet varies from 748 to 668 cm−1 (Jones & Jackson, Reference Jones and Jackson1993) and 750 to 738 cm−1 (Andersen & Brecevic, Reference Andersen and Brecevic1991).
Raman Spectroscopy
Raman spectra were collected using a InVia Raman micro-spectrometer (Renishaw) controlled by the WiRE software (CRC, Paris). Data were collected with a 785 nm laser and a 1,200 lines/mm grating, within the spectral window 100–1,600 cm−1, using 1% of the laser power. Another series of spectra was acquired using a 532 nm laser and a 1,800 lines/mm grating from 100 to 1,600 cm−1 with 0.1% of the laser power. For each analyzed spot, data were scanned for the acquisition of up to 10 spectra and 10 s laser exposure time. Spot positioning was made using a 50× objective lens, giving an analytical spot size of approximately 2 μm in diameter. Spectra were then normalized and averaged. Spectral peak positions were calibrated with a silicon standard. Spectra baseline was corrected using the Crystal Sleuth routine and the wavenumbers were identified by Spectragryph (F. Menges, optical spectroscopy software, http://www.effeOctober 3, 2019mm2.de/spectragryph/).
Calcite peaks are located at 156 and 282 cm−1 (lattice mode), ν4 at 711 cm−1, ν1 at 1,087 cm−1, and ν3 at 1,435 cm−1. Aragonitic peaks are at 152, 182, 209, 217, and 275 cm−1 (lattice mode), a ν4 doublet at 702–706 cm−1, ν1 at 1,084 cm−1, and ν3 at 1,462 cm−1 (Urmos et al., Reference Urmos, Sharma and Mackenzie1991; Nehrke et al., Reference Nehrke, Poignet, Wilhelms-Dick, Brey and Abele2012).
Five main regions are experimentally identified in vaterite: lattice mode (L) from 0 to 340 cm−1, ν4 at 666–685 cm−1 and 738–783 cm−1, ν2 at 873–881 cm−1, ν1 at 1,071–1,091 cm−1, and ν3 at 1,421–1,555 cm−1 (De La Pierre et al., Reference De La Pierre, Demichelis, Wehrmeister, Jacob, Raiteri, Gale and Orlando2014).
Electron Backscatter Diffraction
Three small eggshell fragments (~0.5 cm2) were embedded in epoxy resin and subsequently ground and polished using the sequence described in Perez-Huerta & Cusack (Reference Perez-Huerta and Cusack2009). Once the sample was polished, the surface was coated with 2.5 nm of carbon. The electron backscatter diffraction (EBSD) analysis was carried out with an Oxford Nordlys camera mounted on a Field Emission Scanning Electron Microscope (FESEM) JEOL 7000 located in the Alabama Analytical Research Center (AARC) of the University of Alabama. EBSD data were collected with Oxford Aztec 2.0 software at high vacuum, 20 kV, a large probe current of 16A, working distance of 11 mm, and a resolution of 1 μm step size. Finally, data were analyzed using the OIM 5.3 software from EDAX-TSL. In this study, EBSD data are represented by diffraction intensity, mineral phase, and crystallographic maps and pole figures in reference to the {0001} plane of calcite (see also Perez-Huerta & Dauphin, Reference Perez-Huerta and Dauphin2016).
Results and Discussion
The most common nomenclatures used to describe the structure of calcified eggshells are shown in Figure 3, and bold words are the terms used in this manuscript. Overall, despite its thinness, the usual layered structure of avian eggshells exists in the eggshell of Setophaga. It must be noted that in such small dried and old collected fragments, the outer organic cuticle is not always preserved.
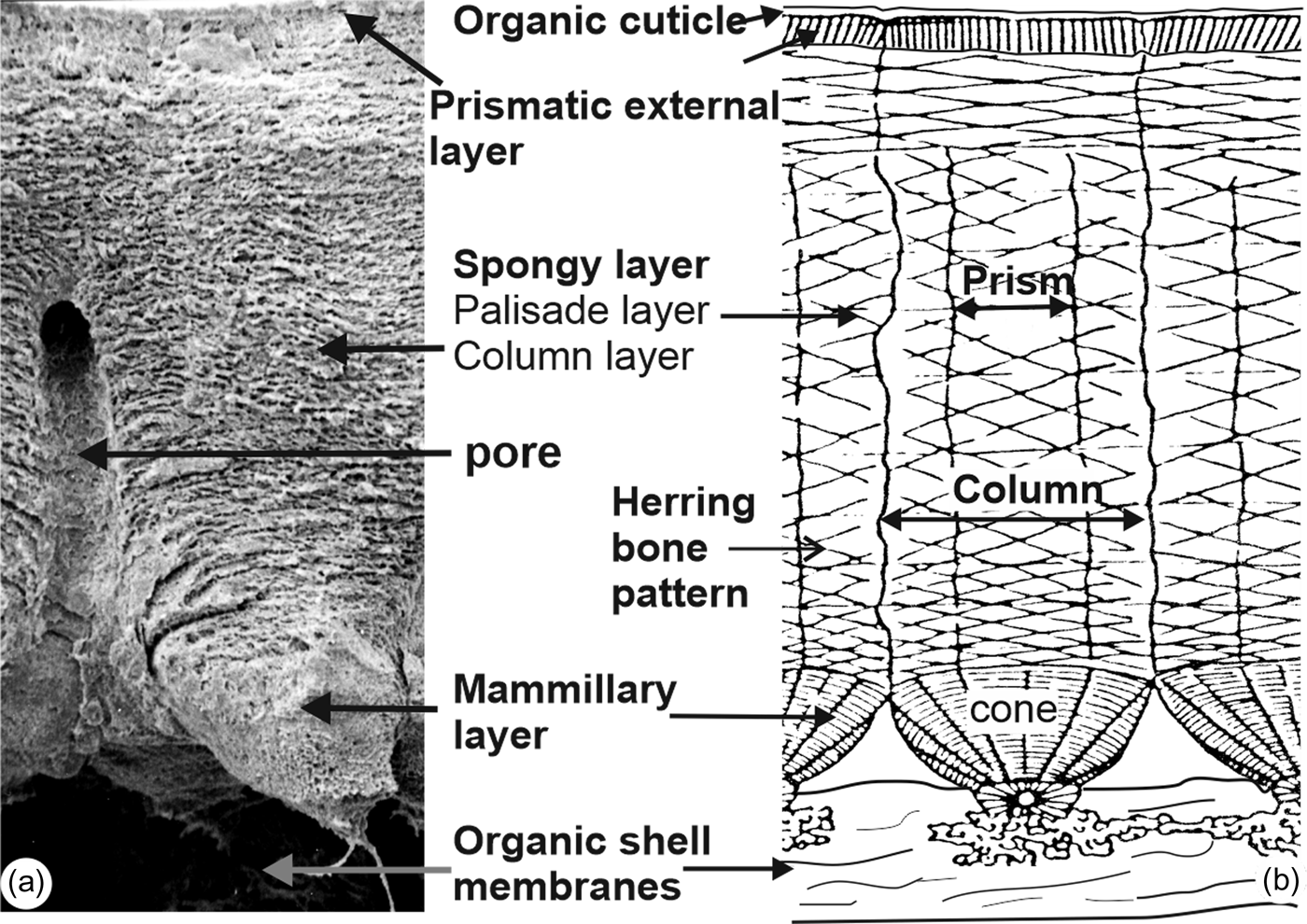
Fig. 3. Main descriptive nomenclatures used for avian eggshells. Bold words are used in this article.
Microstructural Layers (Figs. 4–7)
Three computer tomography (CT) dimensional reconstructions show the main layers of the eggshell (Fig. 4). The irregular outer surface shows a polygonal pattern (Fig. 4a). The palisade and mammillary layers are distinct, but the outer prismatic layer, present in other avian eggshells, is not visible (Fig. 4b). The examination of the inner surface shows the presence of the fibrous organic membrane and the protuberant mammillae (Fig. 4c).
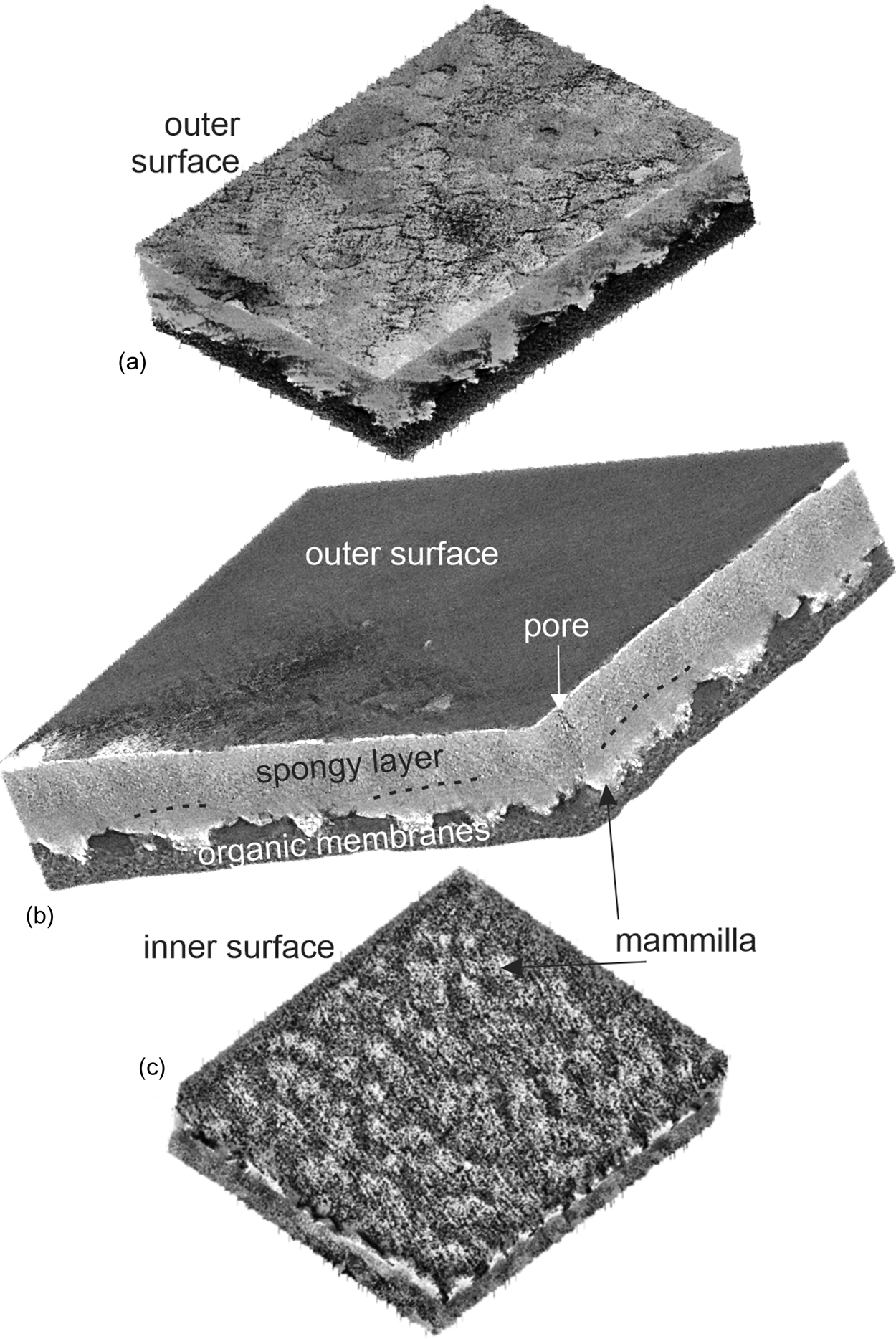
Fig. 4. Three CT dimensional reconstructions showing the main structural features of the eggshell of Setophaga. (a) Outer surface with an irregular polygonal pattern. (b) Oblique view showing the main layers of the eggshell. (c) Inner surface showing the fibrous organic membranes and the calcified mammillae.
Images extracted from tomography data show the irregular polygons and thin cracks of the outer surface of the sample (Fig. 5a). Similar features are also visible in SEM images (Figs. 5b–5d), with the outer organic cuticle being partially destroyed. Pores seem to be oval or round with a single opening (Figs. 5b, 5c). Within the polygons, the orientation of elongated crystallites varies (Fig. 5d). When the organic cuticle is still preserved, the irregular polygonal arrangement is more apparent (Fig. 5e) and the numerous small holes are visible (Fig. 5f).
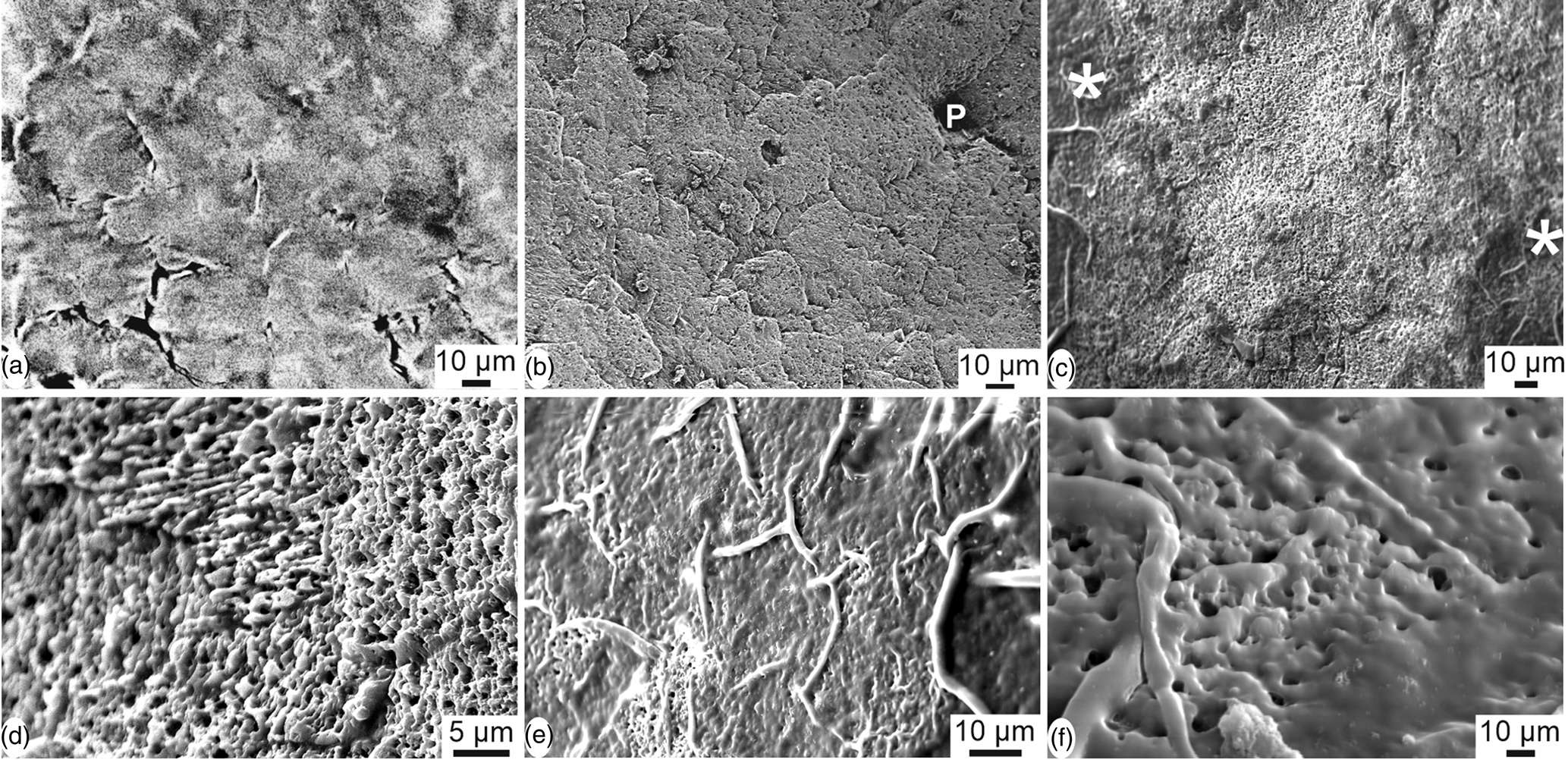
Fig. 5. Outer surface of the eggshell of Setophaga. (a) CT scan reconstruction showing the irregular polygonal pattern of the outer surface; the outer thin organic cuticle is destroyed. (b) SEM image showing the polygonal pattern and a pore (P), the outer pellicle being destroyed. (c) Some polygons and the thin organic cuticle are visible on the left and right sides of the image (*); the organic cuticle is destroyed in the main central part. (d) Detail showing the small acicular crystallites with various orientations, the outer pellicle being destroyed. (e) Polygons are surrounded by an organic bulge when the outer organic cuticle is preserved. (f) Detail of the spongy outer surface still coated with the organic cuticle.
Sections across the eggshell thickness show all the layers known in avian eggshells (Fig. 6): the inner organic membranes (OMs), the spherolites and the mammillary layer (ML), the spongy layer (SL) and pores. Depending on the preservation of the sample, the outer prismatic layer and the organic cuticle are also visible. The thickness of the eggshell is about 40–50 μm. Abundant small rounded vesicles exist in the spongy layer (Figs. 6a–6c, 6e, 6f). Spherolites and mammillae are not contiguous as shown when the inner membranes are partially destroyed (Figs. 6a–6c). The blocky structure is mainly visible in the mammillary layer and in the outer part of the spongy layer (Figs. 6c, 6i–6j). Growth lines and the herringbone pattern usually seen in avian eggshells are not revealed in these sections, but it must be said that these microstructural features are most often described on polished samples. Locally, thin platelets more or less parallel to the outer surface are visible (Fig. 6d). These platelets are an assemblage of elongated acicular crystallites, which are composed of rounded granules (Fig. 6e). Vesicles do not show a preferential orientation (Figs. 6e, 6f). The structure of the prismatic external layer is variable, as shown in Figures 6g–6i: crystallites are sometimes perpendicular to the outer surface of the shell (Fig. 6g). The outer organic cuticle is locally preserved on the outer surface (Fig. 6h). Prisms of the external layer are perpendicular to the surface, as shown in Figure 6g, but they are more compact (Fig. 6i). In some places, granular microcrystals with no visible preferred orientation also exist (Fig. 6j). Vertical sections show that the inner mammillary layer and the spongy layer are always visible, whereas the thin outer prismatic layer and the organic cuticle are variable.
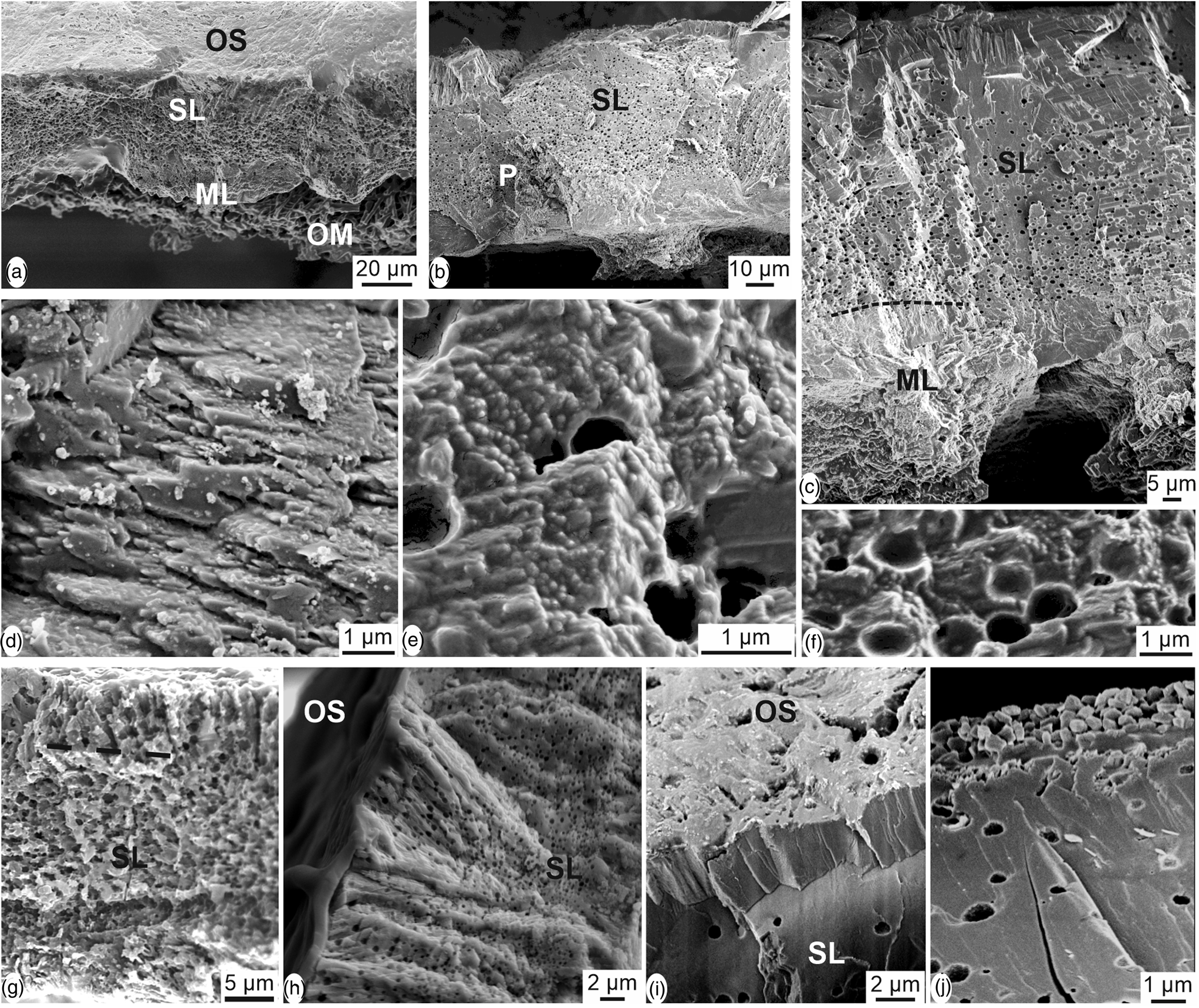
Fig. 6. Vertical sections through the thickness of the eggshell of Setophaga. (a) Fracture showing the large and flat shape of the structural units from the mammilla (ML) toward the outer surface. The outer surface is coated with the very thin organic cuticle as shown by its smooth aspect (OS). Inner organic membranes are locally preserved (OM). (b) Fracture showing the spongy structure of the main layer (SL); the mammillary layer seems more compact and the inner membranes are absent. P, pore. (c) Vertical fracture showing all the mineral layers of the eggshell. Round vesicles are not visible in the mammillary layer (ML). (d) Thin platelets parallel to the outer surface of the eggshell. (e) Elongated crystallites composed of rounded granules. (f) Round vesicles in the spongy layer. (g) Boundary between the spongy layer (SL) and the thin outer prismatic layer is not sharp. (h) The outer organic cuticle is preserved on the outer surface (OS); (i) diverse aspects of the outer part of the spongy layer and the external prismatic layer, depending on the preservation of the dried eggshells.
Oblique CT scan reconstruction show the layered arrangement of the inner part of the eggshell and emphasize the difference between the organic and mineral components (Fig. 7a). The inner fibrillar organic membranes are dark, whereas the spherolitic structures of isolated calcified mammillae are white. Depending on the preservation, the fibrillar membranes attached at the basal part of the mammillae are more or less abundant (Figs. 7b, 7c). The size and shape of the spherolites in the mammillary layer are variable. The inner surface and vertical view show the fibers of the inner organic membranes anchored in the calcareous cone structures (Figs. 7d, 7e). The arrangement of the smooth organic fibers is not compact (Fig. 7f).
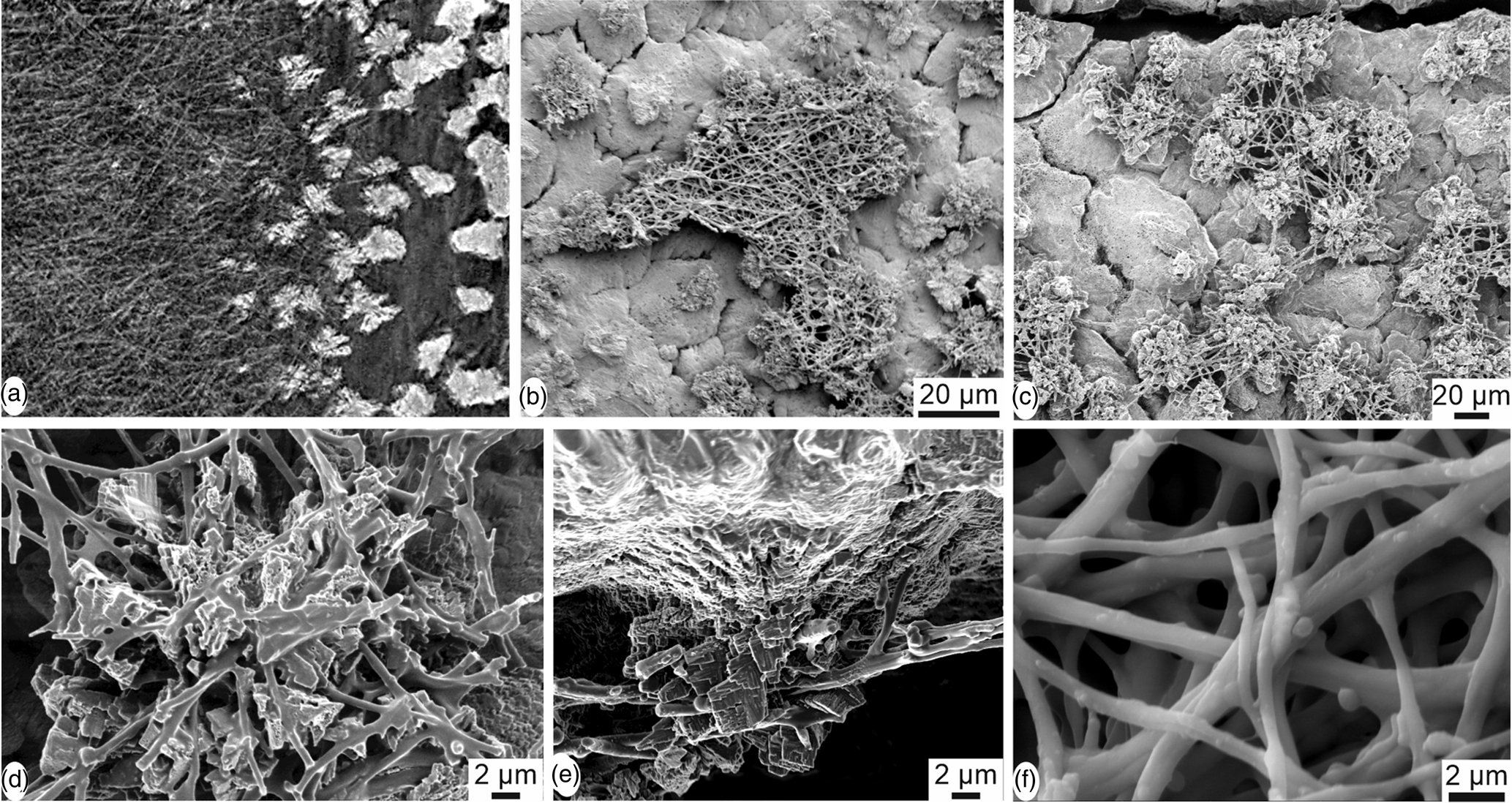
Fig. 7. Inner surface of the eggshell of Setophaga. (a) CT scan oblique reconstructed slice showing the fibrous organic membrane (dark) and the calcified dispersed mammilla (white). (b) SEM image showing a piece of the fibrous membrane. (c) Partially destroyed organic membrane showing the first calcified layers of the mammillary layer; the shape and size of the mammillary units are variable. (d) Remains of the fibrous membrane anchored in the mammilla. (e) Detailed view of the insertion of the organic fibres in the calcareous mammilla. (f) Nodes and smooth aspect of the organic fibres.
Mineralogy
Mineralogy has been analyzed using FTIR and Raman spectrometry. FTIR is sensitive to H2O and CO2 present in the atmosphere and sample, whereas Raman is sensitive to fluorescent compounds.
For FTIR characterization, the strongest band in calcite and aragonite is ν3, between 1,400 and 1,500 cm−1 (Supplementary Fig. S3a). Bird eggshells are composed of mineral and organic matrix as shown by the spectra acquired on the outer and inner surfaces of Setophaga eggshells. The difference between the spectra of the outer surface can be assigned to some difference in color, but the strong difference between the outer and inner surfaces is due to the remains of the inner organic membranes (Supplementary Fig. S3b). Moreover, there is a large overlap in the ν3 region (1,400–1,500 cm−1) between bands assigned to calcite, aragonite, and some organic components (Supplementary Fig. S2b). So, only ν2 and ν4 bands are used to discriminate between the minerals (Fig. 8).
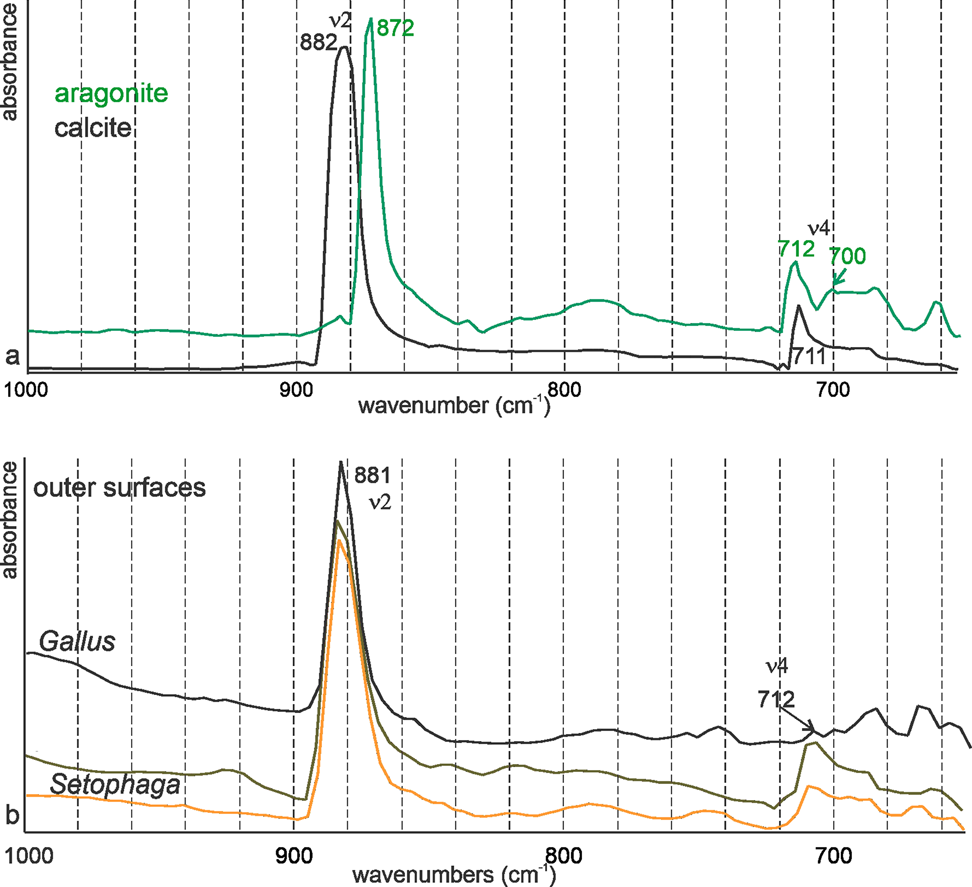
Fig. 8. FTIR spectra of nonbiogenic calcite and aragonite (a) and of the surfaces of Gallus and Setophaga (b). Both eggshells are calcite.
Nonbiogenic calcite and aragonite spectra clearly show the difference in the wavenumbers of ν2 band (872 cm−1 for aragonite and 882 cm−1 for calcite) (Fig. 8a). In aragonite, ν4 is a doublet (Fig. 8a). Spectra acquired on the outer surface of the eggshells of domesticated chicken and Setophaga show that the ν2 band is at 882 cm−1, indicative of calcite (Fig. 8b). In all samples, ν4 is a single band at 712 cm−1, as for calcite. Bands usually assigned to vaterite are absent.
Nonbiogenic calcite and aragonite Raman spectra are displayed in Figure 9a. ν1 (1,080–1,090 cm−1) and ν4 (700–720 cm−1) peaks are “internal modes” due to vibrations between C and O of CaCO3. “Lattice” or “external” modes in the region 100–400 cm−1 are absent in ACC and differ in crystalline CaCO3 (Fig. 9a; Gierlinger et al., Reference Gierlinger, Reisecker, Hild and Gamsjaeger2013; Ramesh et al., Reference Ramesh, Melzner, Griffith, Gobler, Rouger, Tasdemir and Nehrke2018). Both samples show the peaks characteristic for these polymorphs, but aragonite also comprises some peaks probably due to the presence of organic components. Peaks at 1,210 cm−1 and 1,245 cm−1 are known in mollusk shells (Thompson et al., Reference Thompson, North, White and Gallager2014), and peaks at 1,334–1,336 cm−1 are described in the nacreous layers of several bivalve species (Zhang et al., Reference Zhang, Xie and Wang2001). The presence of organic components is not rare in nonbiogenic aragonite (Dauphin & Perrin, Reference Dauphin and Perrin1992).
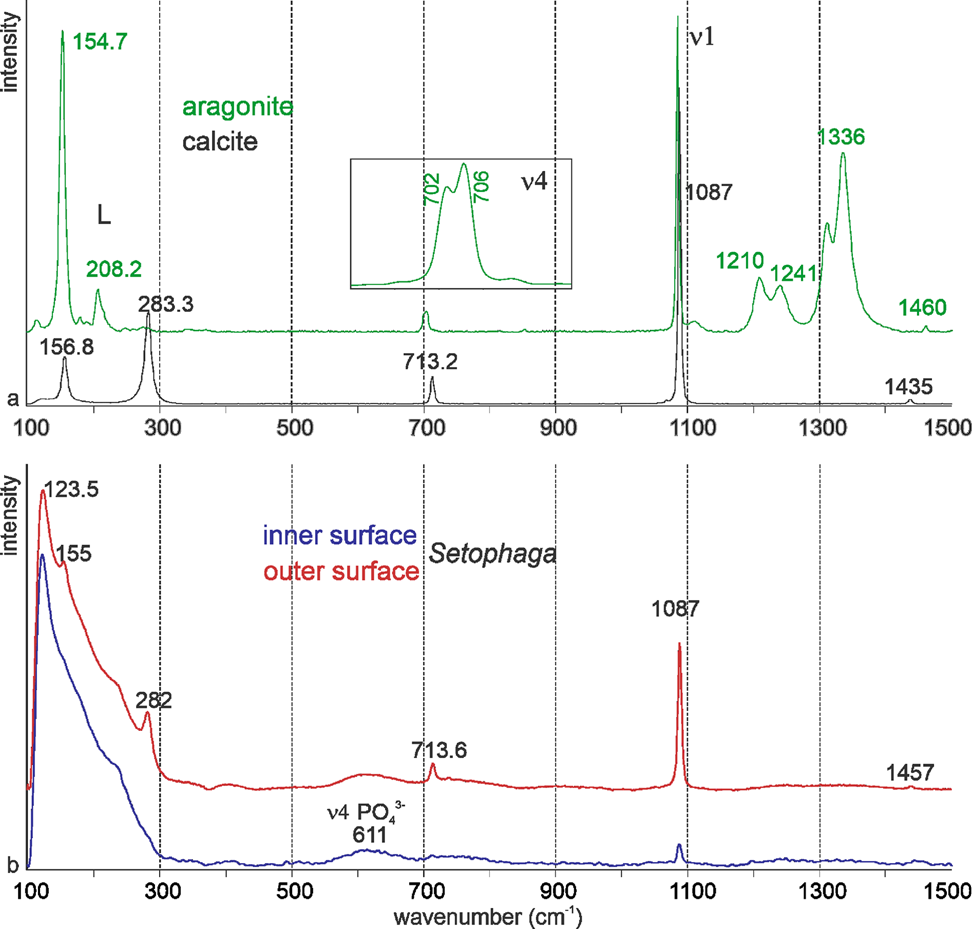
Fig. 9. Raman spectra of nonbiogenic calcite and aragonite (a) and of the surfaces of Setophaga (b), indicative of calcite.
Raman spectra of the outer and inner surfaces of the eggshell are similar and indicative of calcite. The strong broad peaks from 100 to 300 cm−1 are the characteristics of amorphous or poorly crystalline calcium carbonate (Neues et al., Reference Neues, Hild, Epple, Marti and Ziegler2011). A bump near 610 cm−1 visible in both surfaces can be assigned to phosphate and/or proteins. Some peaks are weaker or are visible as bumps in the spectrum of the inner surface (282 and 713 cm−1), due to the presence of remains of the inner organic membranes. The broad peak from 60 to 290 cm−1 is the characteristic of unstable amorphous calcium carbonate.
Crystallography
The EBSD analyses provide information about mineralogy and crystallographic orientations in relation to the microstructure. These analyses were conducted across the eggshell thickness, although most of the inner organic membranes and the mammillary layer were lost during sample preparation due to the shell thickness and fragility. Most of the EBSD data corresponds to the spongy layer, with some information from the very thin prismatic external layer that shows crystallographic continuity (Fig. 10). Although calcite, aragonite, and vaterite phases were added to the analysis, only the presence of calcite was detected. The spongy layer has high crystallinity with a prismatic-blocky structure, and weak overall calcite c-axis oriented perpendicular to the outer surface (Fig. 10).
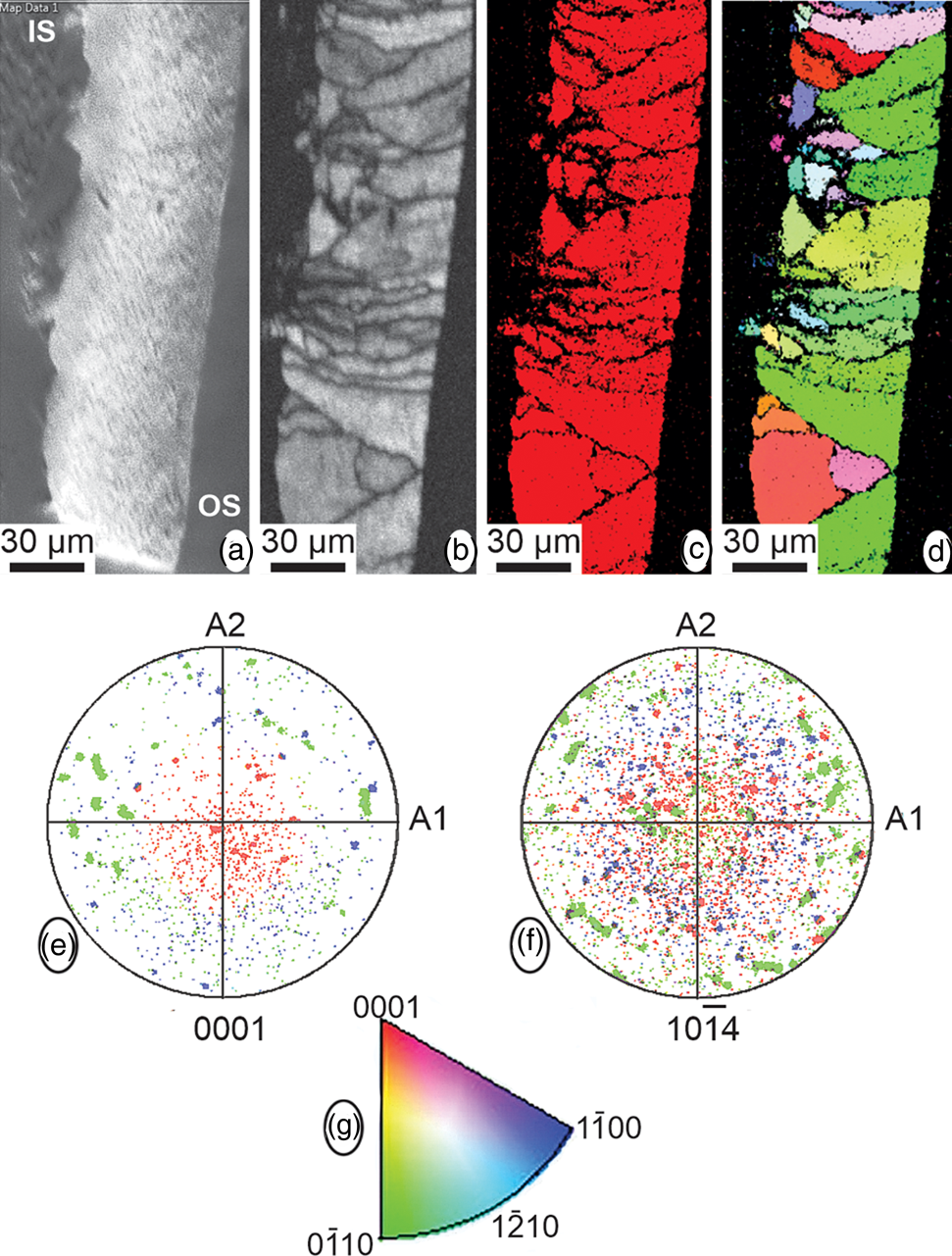
Fig. 10. EBSD data. (a–d) Maps showing, from left to right, the SEM image across the eggshell thickness (IS, internal shell; OS, outer shell), the diffraction intensity map, the phase map, with only one color (red) corresponding to calcite, and the crystallographic map. (e,f) Pole figures, in reference to the {001} and {104} planes of calcite, for the crystallographic map. (g) Color-key legend for the interpretation of the orientation of crystallographic planes in crystallographic map and pole figures.
Conclusion
Our study shows that the porous microstructure of the eggshell of Setophaga is similar to that of other birds with the following arrangement: inner organic fibrous membranes, the mammillary layer with spherolitic arrangement, the palisade layer with numerous small vesicles, a thin outer prismatic layer, and the outer organic cuticle. FTIR and Raman spectrometry data show that the eggshell is calcite. Neither aragonite nor vaterite has been detected. From Raman spectra, amorphous calcium carbonate seems present, but ACC is not visible in the FTIR spectra. EBSD results confirm these results. The overall crystallographic arrangement, with less order and control of the calcite a-, b-, and c-axes, is similar to that previously described for quail and pheasant eggshells (Dalbeck & Cusack, Reference Dalbeck and Cusack2006).
Variability of the mineralogy of carbonate skeletons is rare within a taxon, as, for example, all echinoderm skeletal components are calcitic. Nevertheless, some exceptions are well-known. Among them, a classical example is the aragonitic skeleton of an Heliopora octocoral (also called blue coral), whereas the skeletons of other species within the Subclass Alcyonaria are calcitic. This supports the idea that the mineralogy in biomineralized structures is a conserved phylogenetic feature. Thus, the same mineralogy and overall microstructural arrangement are expected for avian eggshells. Kyser et al. (Reference Kyser, Radcliffe and Langin2007) described the finding aragonite in Setophaga eggshells contradicted our knowledge of biomineralization processes in the context of phylogeny. However, we could not find the presence of aragonite in our study and the mineralogy (calcite) and structural arrangement of Setophaga eggshells are similar to those of other birds. These results support the phylogeny proposed by Erben (Reference Erben1970), and the fact that Setophaga is a Parulidae (Lovette & Bermingham, Reference Lovette and Bermingham2002).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1431927621000301.
Acknowledgments
The authors are grateful to two anonymous reviewers and the editor for their detailed and insightful comments that have significantly improved the manuscript. This study utilized resources owned and maintained by the Alabama Analytical Research Center (AARC), which is supported by The University of Alabama (USA). SEM and CT scan analyses were done using facilities of the Max Planck Institute of Colloids and Interfaces (Potsdam, Germany). Infrared and Raman data were obtained using resources of the Museum national d'histoire naturelle (CRC USR 3224, Paris, France).
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.