Introduction
Fluorescence stereo microscopes are a great and wondrous technology with light sources and optics that can be configured to illuminate a wide array of fluorophors with high sensitivity. There are many applications, however, where a user does not really need all of the power and versatility of a specialized integrated instrument: a simple system that excites just one or two classes of fluorophors will suffice.
NIGHTSEA™ is a small company that arose out of the author's love of diving at night to observe and photograph naturally occurring fluorescence in the underwater environment. The company offered the equipment (special flashlights and filters) that enabled all divers to share this experience, with applications for sport divers, underwater photographers, and marine scientists. Because the fluorescent proteins widely used in biotechnology are of the same family that produce the fluorescence in corals and anemones, it was natural for NIGHTSEA™ to branch out into providing equipment for the laboratory. The new Stereo Microscope Fluorescence Adapter (SFA) system described here is a recent addition to the company's line of equipment.
Need for a new product. Baby corals were the original motivation for development of a low-cost approach to adding fluorescence to existing stereo microscopes. Scientists studying the ecology and health of coral reefs need to better understand recruitment, the process by which new corals become established on the reef. The reef is an extremely complex 3D environment, and newly settled corals are on the order of 1 mm in diameter. This makes them difficult to find in the natural environment. Most corals, however, contain fluorescent proteins, and use of appropriate lights at night makes it fairly easy to spot even the smallest specimens [Reference Piniak, Fogarty, Addison and Kenworthy1, Reference Baird, Salih and Trevor-Jones2].
Because of the difficulty of finding recently settled corals in the natural environment, scientists often deploy small ceramic tiles on the reef for months at a time, bring them back to the lab, and examine them under a conventional reflected-light stereo microscope. In the course of our initial research, with collaborator Dr. Alina Szmant of the University of North Carolina-Wilmington, we investigated whether it would be more efficient to use fluorescence. The answer, as seen in Figure 1, was a resounding “yes.” With white light illumination, corals were hard to see because of low contrast or overhanging algae, whereas fluorescence really made them “pop.”

Figure 1: Juvenile corals on a settlement title viewed in (a) white light and (b) fluorescence with Royal Blue excitation. In addition to the brightly fluorescing coral at the top just right of center, two less intense specimens can also be seen. The general background red fluorescence originates from chlorophyll in algae. Images courtesy of Dr. Alina Szmant, University of North Carolina Wilmington. Image width = 20 mm.
Fluorescence stereo microscope adapter development. The results were clear but not very useful to the reef community. The expense of fluorescence stereo microscopes or even of fluorescence conversion kits for conventional stereo microscopes made them largely unobtainable for coral reef scientists, who need to operate in some of the most remote and least developed parts of the world. A large part of the expense of commercial integrated systems was related to their versatility; they are designed to be general-purpose fluorescence instruments. What if you don't want to solve every fluorescence problem? What if you just want to replicate the excitation light plus barrier filter combination used in underwater flashlight systems? This led to the idea of making a single-purpose rather than general-purpose tool. Just about every marine lab in the tropics has at least one old stereo microscope; it would be possible to take advantage of that resource if fluorescence could be added at reasonable cost.
NIGHTSEA also had been providing flashlights and filter glasses to laboratories that worked with green- and red-fluorescent transgenic animals such as mice. Because the primary fluorescence in corals comes from proteins of the green fluorescent protein (GFP) families, the same basic optical solution worked in both applications. At the urging of Dr. Jason Duncan, a Drosophila researcher at Willamette College, the author redesigned the microscope illuminator concept for use in examining insects. Armed only with two prototypes and a vial of green-fluorescent GFP fruit flies, NIGHTSEA exhibited at the annual Drosophila Research Conference. There was no product to sell, but two primary questions to answer: Do you want this? and Is this what you want? That is, did they want the capability to adapt existing stereo microscopes for fluorescence at low cost, and was the implementation in front of them (blue excitation for green fluorescence) what they wanted. The answer to the first question was yes, and to the second no—they needed to be able to work with multiple colors of fluorescence, primarily green, yellow, and red. So it was back to the drawing board to design what was introduced as the NIGHTSEA™ Stereo Microscope Fluorescence Adapter (SFA).
The design goals for the system were to (a) be as nearly universal as possible, including retrofit to old systems; (b) be useable with both Greenough and common main objective (CMO) designs; (c) require no disassembly or modification of the microscope; (d) be simple to install and remove for easy switching between microscopes; (e) cause no interference with white-light use; (f) allow easy switching between excitation/emission combinations; (g) provide the ability to add new wavelength capabilities at any time; and (h) provide good performance with a wide variety of fluorescing subjects. This article describes the present commercial system, called NIGHTSEA™ SFA, and its applications.
Materials and Methods
At its core any fluorescence system has two basic elements, excitation and emission. The “right way” to do fluorescence excitation in a stereo microscope is to couple the light through the objective lenses (epi-illumination). This is the most efficient way to deliver light to the object. This is what is used in purpose-built fluorescence systems and in most fluorescence conversion systems. Because the excitation light is passing through the objectives, it is necessary to provide an emission barrier filter in the viewing path above the objectives to remove reflected excitation light. The usual solution is the familiar filter cube set that carries complementary excitation and emission filters. By its nature this approach tends to be microscope-specific and in the case of conversion systems requires disassembly of the microscope to insert the new components. This precludes universality and easy switching from one microscope to another. These issues have been addressed in the NIGHTSEA SFA system by taking a simplistic approach that has proven to be effective.
Excitation. The light sources for the SFA are individual light-emitting diode (LED) modules on the end of a flexible support (Figure 2). Each module contains a single high-intensity LED of appropriate wavelength (color), a focusing lens to produce a narrow beam, and a supplementary interference filter. The filter further trims the LED spectral output to achieve the best contrast.

Figure 2: SFA in use with a conventional stereo microscope showing the system components: flexible gooseneck lamp base, modular LED light head, adapter ring, barrier filter, and viewing shield.
The exchangeable excitation module plugs into a gooseneck lamp base that contains a circuit delivering a controlled current to the LED. This approach makes it easy to switch from one wavelength excitation module to another. At its introduction the SFA included a Royal Blue excitation module for exciting GFP and fluorophores such as fluorescein, a Cyan module for exciting yellow-fluorescent proteins and dyes (YFP, Venus, Lucifer yellow, etc.), and a Green module for red fluorescence (DsRed, RFP, mCherry, Tomato, etc.) as shown in Table 1. A Violet excitation module for blue and cyan fluorescence has recently been made available. In addition there is a white light LED module allowing the system to provide general-purpose illumination.
Table 1: Excitation lamp and filter set combinations.

The use of LED excitation results in very low power consumption, and the unit can even be powered from a 12 V battery for off-grid operation. Other advantages of LEDs are that they do not require warm-up or cool-down and will last for 10,000 hours before losing significant intensity.
Emission. Each emission barrier filter is made from optical-grade polycarbonate, selected for its ability to block the wavelength of the complementary excitation source. The filters are longpass filters rather than narrow bandpass (Table 1). This results in little-to-no “crosstalk” between excitation and emission and high viewing contrast.
Emission filters attach to a custom-made adapter that mounts to the microscope by means of thumbscrews, in the same way that a ring light would attach (Figure 3). This adapter is the only part of the SFA system that touches the microscope, and it fits just about every microscope in common use. The user may remove and replace the barrier filter beneath the adapter, where the filter is held in place by magnets. This makes it easy to switch barrier filters when switching fluorophors (Figure 4). For white-light imaging, the user simply inserts the white-light head on the gooseneck and removes the barrier filter without removing the adapter.

Figure 3: The SFA adapter ring has an inside diameter of 67 mm, enabling it to fit most stereo microscopes without a special adapter. The thumbscrews can adjust to fit microscopes with diameters down to 50 mm. The adapter ring holds four small neodymium magnets for attachment of the barrier filters.

Figure 4: The three modular light and filter set combinations originally available for the SFA system (Royal Blue, Cyan, Green). Each set consists of an LED light head, complementary barrier filter, and viewing shield.
The adapter also holds a viewing filter shield made from the same material as the barrier filter. This mounts at a 45-degree angle on the front of the adapter and is secured by a thumbscrew. This filter shields the user from exposure to the excitation light while working at the microscope, reducing eyestrain. In many cases the user can simply look through this viewing shield to see whether or not there is fluorescence in the subject.
In summary, the full SFA system consists of LED excitation modules that mount on a flexible gooseneck base, an adapter that attaches to the microscope below the objectives, barrier filters that attach magnetically to the adapter, and viewing shields that attach to the adapter. It takes about 20 seconds to switch from one color set to another by removing the light head, barrier filter, and viewing shield for one color set and replacing them with the components for the next color set.
Results
The SFA system has been used in a wide variety of applications and has the potential to be applied to many more. The following results are a sampling of these applications.
Screening. Fluorescent proteins are widely used as reporters of gene expression and play a valuable role in many fields of biological research. There are a variety of ways to introduce fluorescent proteins to organisms, but the process is not always successful. Researchers can use the SFA to quickly check if fluorescence is being expressed in any of the typical small model organisms—Drosophila, zebrafish, C. elegans, Xenopus, etc. (see Figure 5).

Figure 5: Example fluorescence images of commonly used model organisms. (a) Zebrafish (Dania rerio) embryo expressing Dendra2 in nuclei (histone H2B Dendra2). Specimen courtesy of Schier Lab, Harvard University (Royal Blue excitation). (b) C. elegans with YFP. Specimen courtesy of Morimoto Lab, Northwestern University (Cyan excitation). (c) Stage 46 Xenopus laevis, messenger RNA injected membrane RFP. Specimen courtesy of National Xenopus Resource, Woods Hole (Green excitation). (d) Drosophila larva expressing GFP driven by an actin promoter. Specimen courtesy of Dr. Laura Reed, University of Alabama Tuscaloosa (Royal Blue excitation). All image widths are approximately 7 mm.
Sorting. Once the gene for fluorescence has been established in a line of organisms, its passage to the next generation may be uncertain. It is often necessary to sort the juveniles into fluorescing and non-fluorescing variants in order to select the ones that are carrying the traits of interest for ongoing experimentation. This is a simple task that is generally well within the capabilities of the SFA and frees up high-end research-grade fluorescence microscopes for more demanding jobs.
As an example, Dr. Laura Reed of the University of Alabama Tuscaloosa was funded for an experiment with Drosophila that would involve a very large number of specimens—enough that the sorting step would easily tie up multiple fluorescence microscopes (not covered by the funding) for extended periods. Dr. Reed saw the prototype SFA at the Drosophila Research Conference and promptly purchased four units equipped with the light and filter sets for use with GFP. She now has multiple undergraduate students using these systems installed on available conventional stereo microscopes to support the research (Figure 6) [Reference Reed, Williams, Springston, Brown, Freeman, DesRoches, Sokolowski and Gibson3].

Figure 6: Student at University of Alabama Tuscaloosa sorting Drosophila. Image courtesy of Laura Reed.
Micro-injection. Dr. Rob Olberg of Union College is researching sensory control of behavior in insects. He studies visual neurons that direct flight in the dragonfly using a combination of techniques ranging from single neuron recording and dye injection to high-speed video analysis of flight behavior. Dr. Olberg added the NIGHTSEA SFA to the microscope he uses to guide the injection of Lucifer yellow dye into axons, making it easier to monitor the process [Reference Frye and Olberg4].
Dissection. Fluorescence, introduced either via fluorescent proteins or other techniques, is often used to label features—organs and growths—that must be removed from the host for more detailed examination or analysis. The SFA has been used on conventional stereo microscopes to illuminate the specimen for dissection. Dr. Xin Lu, a researcher at the MD Anderson Cancer Center in Houston, showed the usefulness of fluorescence during dissection in his work with GFP-tagged tumors in a universally red-fluorescent (RFP) mouse (Figure 7).
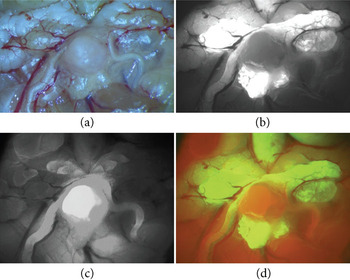
Figure 7: Fluorescence analysis during dissection of GFP-tagged tumors in a universally red-fluorescent (RFP) mouse. (a) white light, (b) using the Royal Blue excitation/emission set to capture the green fluorescence, (c) using the Green excitation/emission set to capture the red fluorescence, and (d) a color composite of the green and red channel images. Images courtesy of Xin Lu, MD Anderson Cancer Center, Houston.
Marine science. The SFA system is of course useful for the application for which it was first developed: coral recruitment research. There are many other potential applications in marine science. Dr. Randi Rotjan of the New England Aquarium has used the system to study the gut contents of fish that feed in the coral reef environment. The system also has been used on board a research vessel to examine an intensely fluorescing ostracod discovered during SCUBA explorations in the Bahamas (Figure 8) [Reference Jarett, Fiore, Mazel and Lesser5].

Figure 8: An ostracod (bivalve crustacean) with intensely fluorescent carapace discovered during SCUBA explorations in the Bahamas. The fluorescence arises from an epibiotic bacterial community. White light (a) and fluorescence (Royal Blue excitation) images. Image width = 3 mm.
Exploration of nature. Many examples of naturally occurring fluorescence can be found in the environment. Having a simple fluorescence microscopy system at hand makes it easy to explore this spectral dimension of the world around us. The photographs in Figure 9 show fluorescence in a small plant burr (the kind that sticks to clothing) and in the hairs on the abdomen of an ant. Because of depth of field limitations, focus stacking [Reference Piper6] was used to create these images.

Figure 9: Naturally occurring fluorescence in (a) a small plant burr removed from clothing and (b) hairs on the abdomen of an ant. Focus stacking of multiple images was used to create these images. Both images were made with Royal Blue excitation. Image widths 6 mm and 4 mm, respectively.
Education. The SFA system is proving popular for educational applications. When the system was first exhibited, quite a few researchers said, “Now I can teach a genetics course using [your favorite animal model here].” Dr. Jason Meyers of Colgate University purchased two systems for classes and had this to say: “Students in Developmental Biology Lab were examining the effects of pharmacological agents on the development of zebrafish embryos. In order to better visualize the development of the nervous system and vasculature, we used transgenic fish that expressed GFP either throughout their nervous system or in the developing vasculature. The NIGHTSEA system easily adapted to our dissection scopes and allowed students to observe the development of their fish at several different time points. They could readily observe the transgene expression; it helped solidify the phenotypes they were observing. It allowed them to determine an optimal time to fix their fish for analysis under the compound microscope. For quick screens it actually worked well in a bright room. For more intimate examination (more than presence/absence calls), we turned out the room lights. Worked better than I'd hoped it would.”
Several universities have acquired upwards of 25 systems to outfit the full complement of stereo microscopes in a teaching laboratory. The total cost of those systems was less than the cost of a single research-grade fluorescence stereo microscope.
This system also can bring fluorescence microscopy to biology classes in schools. Dr. Michael Barresi of Smith College runs an outreach program teaching genetics in middle schools using fluorescent zebrafish and now uses the SFA to bring fluorescence microscopy with him on the road.
Lab startups. The SFA system has been popular with early-stage researchers leaving a postdoc for their first academic position. They are generally allocated limited funds with which to jump-start their research. If their work involves fluorescent transgenic models such as zebrafish, C. elegans, or Drosophila, a conventional fluorescence microscope could consume a large portion of those funds. The SFA enables them to get a running start on their research program with a workhorse tool that will do most of what they initially need.
Non-destructive testing. Non-destructive testing (NDT) encompasses a broad group of analysis techniques used in science and industry to evaluate the properties of a material, component, or system without causing damage. Two NDT techniques, fluorescent dye penetrant and magnetic particle inspection, cause a fluorescent indicating material to localize at surface features, which may be defects that would not otherwise be visible (Figure 10). Although these techniques are usually applied at a macroscopic scale, microscopic investigation can be useful when cracks or other features need to be examined in detail to address engineering problems.

Figure 10: Fluorescent penetrant indicating fine cracks in a steel plate under Royal Blue excitation. Image width = 20 mm.
Circuit board repair. Printed circuit boards consist of copper conductor traces on fiberglass board material. The fiberglass fluoresces green, but the copper is entirely non-fluorescent, so it appears black. Figure 11 shows how the SFA helped a technician make repairs to a printed circuit board with an erroneous trace that needed to be cut. There was much better contrast in fluorescence than in white light, making it easier to see when the copper had been cut all the way through.

Figure 11: Section of a circuit board in (a) white light and (b) fluorescence. The board itself is fluorescent, and the copper trace under the silk screen layer is not. The resulting high contrast facilitated repair of the board. The cutting of the trace is more evident in fluorescence. Royal Blue excitation. Image width = 4 mm.
Conclusion
The use of fluorescence microscopy is widespread and growing in science and engineering, but it can be expensive to acquire a dedicated fluorescence microscope. Simple stereo microscopes are inexpensive and nearly ubiquitous. Add-on adapters can convert most existing stereoscopes for fluorescence work at modest cost. Using careful control of excitation and emission, the system can provide fluorescence intensity and contrast that are more than adequate for a wide range of applications.














