Introduction
The Lower Permian N.E. Frederick fossil site (Sam Noble Oklahoma Museum of Natural History V 173, Tillman County, OK), which is located 47 miles from Richards Spur, OK, yielded permineralized synapsid femur, jaw, tooth, and rib specimens (Paleozoic) in May of 2021 (Figures 1–5) that were subjected to a petrographic study. Site V 173 is noted for yielding exceptional Lower Permian bones from fish, sharks, amphibians, and reptiles from over 28 genera, including Dimetrodon [Reference Cope1–Reference MacDougall5].

Figure 1: Dimetrodon femur, in situ, N.E. Frederick, OK.

Figure 2: Portion of Dimetrodon femur sent for sectioning.

Figure 3: Portion of Dimetrodon jaw sent for sectioning.

Figure 4: Portion of Dimetrodon tooth sent for sectioning.

Figure 5: Portion of Dimetrodon rib sent for sectioning.
Skeletal remains of Paleozoic terrestrial animals have been recovered from Illinois [Reference Cope6], Utah [Reference Vaughn and James7], and Europe [Reference Berman8], however, the best sites are in Oklahoma [Reference MacDougall5] and Texas [Reference Cope9–Reference Shelton17]. Synapsid specimens such as Dimetrodon are rare worldwide, but of Oklahoma it has been said, “tens of thousands of [Paleozoic] collected specimens” are preserved from one site alone, Richards Spur [Reference Evans3]. Dimetrodon, the subject of this study, has been collected widely across North America [Reference Cope1,Reference Cope6,Reference Vaughn and James7,Reference Cope9–Reference Florides15] with most of the 12 known species of Dimetrodon coming from Texas [Reference Huttenlocker16–Reference Agliano20].
Few histological reports of Dimetrodon or other synapsid bones exist, but teeth, long bones, and vertebra have been sectioned and reported [Reference Huttenlocker16–Reference Agliano20]. Those reports are compared to the material presented here: Dimetrodon femur (DSTRI-5-4-G, Figures 1–2), jaw (DSTRI-5-4-Ja, Figure 3), tooth (DSTRI-5-4-Jb, Figure 4, red arrow), and rib (DSTRI-5-4-K, Figure 5). The purpose of this study was to examine ground sections of Dimetrodon bone for evidence of blood clots in vessel canals.
Materials and Methods
Dimetrodon specimens were collected from pebbly and granular surface deposits on a lower Permian bed near the shoreline of Lake Frederick, OK. Bones were placed into 10% formalin for transport to the lab. A newly sanitized laboratory space was secured and entry restricted to only two technicians. Bones were rinsed in distilled water, air-dried, and specimen ID numbers painted on the reverse. After stabilizing at room temperature for 5 days, ground sections approximately 40 microns thick were coated with a liquid polymer for stability and cured overnight, but not coverslipped. Sections, without coverslips, were viewed with 374 nm UV autofluorescence microscopy for evidence of blood clots in vascular canals, as described by Armitage and Solliday [Reference Armitage and Solliday21].
Results
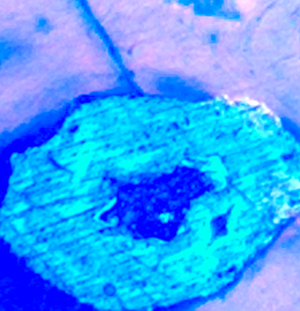
The Dimetrodon femur (DSTRI-5-4-G, Figures 1–2) was approximately 11 cm long and 3 cm wide at mid-diaphysis when located in situ. Severe dorsoventral crushing of the femur must have occurred at death or during burial because multiple 2 to 3 mm wide fractures were preserved through permineralization. Under brightfield examination, reddish-brown opaque masses filled the entire lumen of many vascular canals (Figure 6a). When subjected to UV autofluorescence, the masses fluoresced brightly, as observed previously with ground sections of Cretaceous bone [Reference Armitage and Solliday21] (Figure 6b, de-colorized). More femur-filled vascular canals with glowing masses are seen in Figures 7 and 8. We interpret these masses to be clotted blood, which remained in situ, filling the vessel canals [Reference Armitage and Solliday21].

Figure 6a: Brightfield image of clot in femur vascular canal (red asterisk) and infiltrated calcite (blue asterisks). Note granular nature of clot. Scale bar = 100 μm.

Figure 6b: Ultraviolet fluorescence image of clot from Figure 6a (de-colorized). Note bright, uniform autofluorescence signal from iron in clot (red asterisk). Crystallized blood products are the dark inclusions within the clot. Scale bar = 100 μm.

Figure 7a: Ultraviolet fluorescence and brightfield combined image of Haversian canal with femur clot blocking the entire lumen. A bright line of demarcation at the outer edges of the clot (black arrows) shows that the iron did not escape the canal. Deeper portion of the clot extending downward into the canal (red arrow). Scale bar = 80 μm.

Figure 7b: Ultraviolet fluorescence image of the clot from 7a. Scale bar = 80 μm.
Jaw (DSTRI-5-4-Ja, Figures 9a, 9b), tooth (DSTRI-5-4-Jb, Figures 10a, 10b, and 11a, 11b) and rib (DSTRI-5-4-K, Figures 12a, 12b and 13, 14) ground sections likewise exhibited clots, which filled the lumens of most vascular canals and glowed brightly under UV autofluorescence. Straight-line polishing marks are clearly seen across the areas where iron was exposed and polished (Figures 8a, 8b, 11a, 11b, 12b, 13, 14), typical of a malleable metal. Polishing marks are only visible under reflected light (fluorescence, brightfield, or darkfield). A steel pin extended over the field diaphragm of the microscope (Figure 15) demonstrates that the iron in the clot is more dense than the steel pin visible underneath the clotted canal. All bones were deeply infiltrated with calcite, (yellow color in polarized light with a compensator), yet clots remained as clogs in many canals (Figure 16, green arrows). Remaining Dimetrodon material from this study is reposited at DSTRI, Inc., Mansfield, TX.

Figure 8a: Several clots from Dimetrodon femur. Orange arrows indicate the lumen-filling clot extending deeper into the vascular canal below the plane of focus. Note the polishing marks in the malleable metal clot. Scale bar = 60 μm.

Figure 8b: Ultraviolet fluorescence image of the clots from 8a. Scale bar = 60 μm.

Figure 9a: Brightfield image, Dimetrodon jaw. Blood clot filling a Haversian vessel canal. Scale bar = 60 μm.

Figure 9b: Ultraviolet image of the clot from 9a. Scale bar = 60 μm.

Figure 10a: Brightfield image, Dimetrodon tooth, clot filling a Haversian vessel canal. Scale bar = 60 μm.

Figure 10b: Ultraviolet image of the clot from 10a. Scale bar = 60 μm.

Figure 11a: Combined brightfield and ultraviolet fluorescence image, Dimetrodon tooth, clot filling a Haversian vessel canal. Note polishing marks on iron, indicating it is malleable. Scale bar = 60 μm.

Figure 11b: Ultraviolet fluorescence image of the clot from 11a. Scale bar = 60 μm.

Figure 12a: Brightfield image, Dimetrodon rib, clot filling a Haversian vessel canal. Scale bar = 60 μm.

Figure 12b: Ultraviolet fluorescence image of the clot from 12a. Note polishing marks on iron, indicating it is malleable. Scale bar = 60 μm.

Figure 13: Ultraviolet fluorescence image, Dimetrodon rib. Clot filling right side of bifurcated canal. Note polishing marks on iron, indicating it is malleable. Scale bar = 60 μm.

Figure 14: Ultraviolet fluorescence image, Dimetrodon rib. Clot filling Haversian canal. Note polishing marks and that the crack through the bone cleanly split the iron clot. Scale bar = 60 μm.

Figure 15: Ultraviolet fluorescence image, Dimetrodon jaw. Note the steel pin, mounted over the field diaphragm (red asterisks) indicating the thin clot (yellow arrow) is denser than the pin. Scale bar = 60 μm.

Figure 16: Crossed polarized image of Dimetrodon femur. Note infiltrating calcite (red asterisks) and the many clotted canals (green arrows). Scale bar = 275 μm.
Discussion and Conclusions
The surface of site V 173 was littered with varying sizes of pebbles, stones, and bone fragments, ranging from dark to bone-white in color. These shards and pebbles were found touching and surrounding the collected bones (Figure 1). Bones and fragments came away from the surface only with some tugging, as if they were cemented in place. The color of the subsurface material, a non-calcareous, silty clay, was darker than the bleached lighter color of the surface and contained significant moisture due to severe thunderstorms that had been active in the area just a week before collection began.
We compared our brightfield observations of reddish-brown clots in Dimetrodon specimens with histology studies of other Paleozoic bones. Shelton et al. [Reference Shelton17] transversely sectioned D. natalis femora as well as humeri collected from Texas. Cross-section images of those bones showed infiltration of calcite and other minerals, and significant dark and opaque masses remained within each medullary cavity. Brink and Reisz also sectioned D. grandis teeth from Texas [Reference Brink and Reisz18]. We conclude that the dark areas seen on Figure 5D of that work are probably clots. Huttenlocker and Shelton transversally sectioned Dimetrodon sp. humeri from Oklahoma [Reference Huttenlocker and Shelton19]. Although we produced longitudinal sections in Dimetrodon, there is remarkable similarity between our Figure 16 and Figures 3, 5, and 6 of that study. Canals in both studies exhibit the same reddish-brown clots at the center of Haversian systems. Agliano et al. [Reference Agliano20] sectioned Dimetrodon vertebrae and showed deeply clotted canals in their Figures 4 and 5. All of these ground specimens need examination under UV-autofluorescence to confirm our findings. Finally, we note the well-known work by Davis on vertebrate bones from the lower Permian [Reference Davis22]. We counted 45 images of sections of Paleozoic material in that work, which includes Dimetrodon, and many of the images show robust clots. We would especially draw attention to the plates on pages 354–57 as a particularly good example of clots within Dimetrodon teeth and vertebrae.
A discussion of disseminated intravascular coagulation (DIC), which is a clotting condition typical of drowning victims, exceeds the purpose of this paper, as it was detailed in a previous work [Reference Armitage and Solliday21]. These results show that Dimetrodon bones collected in the Lower Permian of Oklahoma display heavy clotting within vascular canals suggesting that DIC was the cause of death, followed by burial and mineral replacement that did not dislodge all clots from vessel canals.
Acknowledgements
Thanks to Bill and Julie May from the Sam Noble Oklahoma Museum of Natural History for site guidance, collection, and identification assistance and for constructive comments. Thanks also to Joe Taylor and Stan Lutz from the Mt. Blanco Fossil Museum, and DSTRI, Inc. personnel for assistance and funding.