Article contents
Veblenite, K2☐2Na(Fe2+5Fe3+4Mn2+7☐)Nb3Ti(Si2O7)2(Si8O22)2O6(OH)10(H2O)3, a new mineral from Seal Lake, Newfoundland and Labrador: mineral description, crystal structure, and a new veblenite Si8O22 ribbon
Published online by Cambridge University Press: 05 July 2018
Abstract
Veblenite, ideally K2☐2Na(Fe2+5Fe3+4Mn2+7☐)Nb3Ti(Si2O7)2(Si8O22)2O6(OH)10(H2O)3, is a new mineral with no natural or synthetic analogues. The mineral occurs at Ten Mile Lake, Seal Lake area, Newfoundland and Labrador (Canada), in a band of paragneiss consisting chiefly of albite and arfvedsonite. Veblenite occurs as red brown single laths and fibres included in feldspar. Associated minerals are niobophyllite, albite, arfvedsonite, aegirine-augite, barylite, eudidymite, neptunite, Mn-rich pectolite, pyrochlore, sphalerite and galena. Veblenite has perfect cleavage on {001} and splintery fracture. Its calculated density is 3.046 g cm–3. Veblenite is biaxial negative with α 1.676(2), β 1.688(2), γ 1.692(2) (λ 590 nm), 2Vmeas = 65(1)°, 2Vcalc = 59.6°, with no discernible dispersion. It is pleochroic in the following pattern: X = black, Y = black, Z = orange-brown. The mineral is red-brown with a vitreous, translucent lustre and very pale brown streak. It does not fluoresce under short and long-wave UV-light. Veblenite is triclicnic, space group P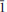 , a 5.3761(3), b 27.5062(11), c 18.6972(9) Å, α 140.301(3), β 93.033(3), γ 95.664(3)°, V = 1720.96(14) Å3. The strongest lines in the X-ray powder diffraction pattern [d(Å)(I)(hkl)] are: 16.894(100)(010), 18.204(23)(0
, a 5.3761(3), b 27.5062(11), c 18.6972(9) Å, α 140.301(3), β 93.033(3), γ 95.664(3)°, V = 1720.96(14) Å3. The strongest lines in the X-ray powder diffraction pattern [d(Å)(I)(hkl)] are: 16.894(100)(010), 18.204(23)(0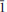 1), 4.271(9)(
1), 4.271(9)(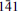 , 040, 120), 11.661(8)(001), 2.721(3)(
, 040, 120), 11.661(8)(001), 2.721(3)(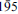 ), 4.404(3)(
), 4.404(3)( ,
, 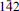 ), 4.056(3)(031, 1
), 4.056(3)(031, 1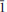 2;
2;  ,
, 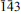 ), 3.891(2)(003).
), 3.891(2)(003).
The chemical composition of veblenite from a combination of electron microprobe analysis and structural determination for H2O and the Fe2+/Fe3+ ratio is Nb2O5 11.69, TiO2 2.26, SiO2 35.71, Al2O3 0.60, Fe2O3 10.40, FeO 11.58, MnO 12.84, ZnO 0.36, MgO 0.08, BaO 1.31, SrO 0.09, CaO 1.49, Cs2O 0.30, K2O 1.78, Na2O 0.68, H2O 4.39, F 0.22, O = F –0.09, sum 95.69 wt.%. The empirical formula [based on 20 (Al+Si) p. f. u. is (K0.53Ba0.28Sr0.03☐0.16)Σ1(K0.72Cs0.07☐1.21)Σ2(Na0.72Ca0.17☐1.11)Σ2(Fe2+5.32Fe3+4.13Mn2+5.97Ca0.70Zn0.15Mg0.07☐0.66)Σ17(Nb2.90Ti0.93Fe3+0.17)Σ4(Si19.61Al0.39)Σ20O77.01H16.08F0.38. The simplified formula is (K, Ba, ☐)3(☐, Na)2(Fe2+, Fe3+, Mn2+)17(Nb,Ti)4(Si2O7)2(Si8O22)2O6(OH)10(H2O)3. The infrared spectrum of the mineral contains the following bands (cm–1): 453, 531, 550, 654 and 958, with shoulders at 1070, 1031 and 908. A broad absorption was observed between ~3610 and 3300 with a maximum at ~3525. The crystal structure was solved by direct methods and refined to an R1 index of 9.1%. In veblenite, the main structural unit is an HOH layer, which consists of the octahedral (O) and two heteropolyhedral (H) sheets. The H sheet is composed of Si2O7 groups, veblenite Si8O22 ribbons and Nb-dominant D octahedra. This is the first occurrence of an eight-membered Si8O22 ribbon in a mineral crystal structure. In the O sheet, (Fe2+, Fe3+, Mn2+) octahedra share common edges to form a modulated O sheet parallel to (001). HOH layers connect via common vertices of D octahedra and cations at the interstitial A(1,2) and B sites. In the intermediate space between two adjacent HOH layers, the A(1) site is occupied mainly by K; the A(2) site is partly occupied by K and H2O groups, the B site is partly occupied by Na. The crystal structure of veblenite is related to several HOH structures: jinshanjiangite, niobophyllite (astrophyllite group) and nafertisite. The mineral is named in honour of David R. Veblen in recognition of his outstanding contributions to the fields of mineralogy and crystallography.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 6
- Cited by


