Article contents
Ammoniotinsleyite, (NH4)Al2(PO4)2(OH)⋅2H2O, a new mineral species from the guano deposit at Pabellón de Pica, Iquique Province, Chile
Published online by Cambridge University Press: 05 June 2020
Abstract
The new leucophosphite-group mineral ammoniotinsleyite is found in a guano deposit located on the Pabellón de Pica Mountain, Iquique Province, Tarapacá Region, Chile. Associated minerals are halite, gypsum, salammoniac and clay minerals. Ammoniotinsleyite occurs as pink to pale violet globular aggregates up to 3 mm across with individual single crystals ~10–15 μm. The mineral is brittle. Its Mohs hardness is 4. Dmeas. = 2.42(2) g cm–3 and Dcalc. = 2.451 g cm–3. The IR spectrum shows the presence of NH4+ and PO43– groups and H2O molecules. Ammoniotinsleyite is optically biaxial (+), α = 1.557(2), β = 1.559 (calc.), γ = 1.563(2) (λ = 589 nm); and 2Vmeas. = 75(10)°. The chemical composition (K, Mg, Ca, Al, Fe and P from electron-microprobe data; H, C and N measured by gas chromatography on products of ignition at 1200°C; wt.%) is: (NH4)2O 7.25, K2O 1.50, MgO 0.42, CaO 0.34, Al2O3 29.91, Fe2O3 2.36, P2O5 43.97, H2O 14.89, CO2 below detection limit, total 100.64. The empirical formula is [(NH4)0.88K0.10Ca0.02)]Σ1.00(Al1.86Fe3+0.09Mg0.03)Σ1.98(PO4)1.96(OH)1.05⋅2.11H2O. The idealised formula is (NH4)2Al2(PO4)2(OH)⋅2H2O. The crystal structure of ammoniotinsleyite was refined based on powder X-ray diffraction data, using the Rietveld method. The final agreement factors are: Rp = 0.0071, Rwp = 0.0093 and Robs = 0.0167. The new mineral is isostructural with tinsleyite, spheniscidite and leucophosphite. It is monoclinic, space group P21/n, a = 9.5871(1) Å, b = 9.6089(1) Å, c = 9.6467(2) Å, β = 103.4461(8)°, V = 864.31(2) Å3 and Z = 4. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I,%)(hkl)] are: 7.56(23)(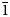 $\bar{1}$01), 6.71(79)(011, 110), 5.947(100)(101,
$\bar{1}$01), 6.71(79)(011, 110), 5.947(100)(101, 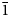 $\bar{1}$11), 4.676(36)(002, 200), 3.032(28)(
$\bar{1}$11), 4.676(36)(002, 200), 3.032(28)(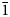 $\bar{1}$13, 031, 130), 2.958(25)(
$\bar{1}$13, 031, 130), 2.958(25)(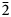 $\bar{2}$22, 310,
$\bar{2}$22, 310, 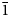 $\bar{1}$31) and 2.635(29)(
$\bar{1}$31) and 2.635(29)(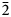 $\bar{2}$31).
$\bar{2}$31).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2020
Footnotes
Associate Editor: Ian T. Graham
References
- 4
- Cited by


