No CrossRef data available.
Article contents
Coquandite, Sb6+x O8+x (SO4)(OH)x ·(H2O)1–x (x = 0.3), from the Cetine mine, Tuscany, Italy: crystal structure and revision of the chemical formula
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of the mineral coquandite, a rare Sb oxy-sulfate hydrate, was solved using intensity data collected from a crystal from the Cetine mine, Tuscany, Italy. This study revealed that the structure is triclinic, space group P 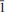 , with a = 11.4292(5), b = 29.772(1), c = 11.2989(5) Å, α = 91.152(3), β = 119.266(4), γ = 92.624(3)° and V = 3346.4(2) Å3. The refinement of an anisotropic model led to an R index of 0.0347 for 21,061 independent reflections. Thirty-two Sb sites, five S sites and 67 oxygen sites occur in the crystal structure of coquandite. Sb atoms display the characteristic SbO3 E and SbO4 E coordinations whereas S fills (SO4) tetrahedral groups. These atoms are arranged in five symmetry-independent layers perpendicular to b*. Four of them and their centrosymmetrical counterparts form complex modules stacked along b* and bonded through two Sb atoms and H bonds. The complex H bonding system in the structure is discussed. On the basis of information gained from this characterization, the crystal-chemical formula was revised according to the structural results, yielding Sb6+x O8+x (SO4)(OH)x ·(H2O)1–x (Z = 10) with x = 0.3 instead of Sb6O8(SO4)·H2O (Z = 12) as reported previously. A recalculation of the chemical data listed in the scientific literature for coquandite according to the structural results obtained here leads to a satisfactory agreement.
, with a = 11.4292(5), b = 29.772(1), c = 11.2989(5) Å, α = 91.152(3), β = 119.266(4), γ = 92.624(3)° and V = 3346.4(2) Å3. The refinement of an anisotropic model led to an R index of 0.0347 for 21,061 independent reflections. Thirty-two Sb sites, five S sites and 67 oxygen sites occur in the crystal structure of coquandite. Sb atoms display the characteristic SbO3 E and SbO4 E coordinations whereas S fills (SO4) tetrahedral groups. These atoms are arranged in five symmetry-independent layers perpendicular to b*. Four of them and their centrosymmetrical counterparts form complex modules stacked along b* and bonded through two Sb atoms and H bonds. The complex H bonding system in the structure is discussed. On the basis of information gained from this characterization, the crystal-chemical formula was revised according to the structural results, yielding Sb6+x O8+x (SO4)(OH)x ·(H2O)1–x (Z = 10) with x = 0.3 instead of Sb6O8(SO4)·H2O (Z = 12) as reported previously. A recalculation of the chemical data listed in the scientific literature for coquandite according to the structural results obtained here leads to a satisfactory agreement.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014


