Published online by Cambridge University Press: 28 February 2018
Embreyite from the Berezovskoe, Urals, Russia, was studied by the means of powder X-ray diffraction (XRD), single-crystal XRD, infrared spectroscopy and microprobe analysis. The empirical formula of embreyite obtained on the basis of microprobe analysis is Pb1.29Cu0.07Cr0.52P0.43O4 (without taking into account the presence of H2O). An examination of single-crystal XRD frames of the tested crystals cut from embreyite intergrowths revealed split reflection spots of weak intensities, even after a long exposure time. The crystal structure of embreyite (monoclinic, C2/m, a = 9.802(16), b = 5.603(9), c = 7.649(12) Å, β = 114.85(3)o and V = 381.2(11) Å3) has been solved by direct methods and refined to R1 = 0.050 for 318 unique observed reflections. The powder XRD patterns of the holotype embreyite and the fresh material studied are close in both d values and the intensities match the pattern calculated from the structural single-crystal XRD data. The unit-cell parameters were re-calculated for the holotype sample using a new cell setting and corresponding hkl indices. The crystal structure of embreyite is based on layers formed by corner-sharing mixed chromate-phosphate tetrahedra and PbO6 distorted octahedra. The interlayer space is filled by disordered Pb2+ and Cu2+ cations. Generally, the crystal structure of embreyite can be referred to the structural type of palmierite. {Pb[(Cr,P)O4]2]} layers in embreyite are similar in topology to those in yavapaiite-type compounds. The general formula of embreyite can be represented as (Pbx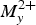 $M_y^{2 +} $□1–x–y)2{Pb[(Cr,P)O4]2}(H2O)n, where M2+ = Cu and Zn and 0.5 ≤ x + y ≤ 1, or, in the simplified form: (Pb,Cu,□)2{Pb[(Cr,P)O4]2}(H2O)n. The simplified formula of embreyite is similar in stoichiometry to vauquelinite and may explain the existence of the solid-solution series. The determination of the crystal structure of embreyite may also help to resolve the crystal chemical nature of cassedanneite. The XRD pattern of cassedanneite contains a distinct reflection with d = 13.9 Å, forbidden for the embreyite unit cell. This feature may indicate the doubling of the c unit-cell parameter of cassedanneite in comparison with embreyite. We assume that cassedanneite has structural similarity to embreyite with, presumably, a disordered distribution of Cr and V.
$M_y^{2 +} $□1–x–y)2{Pb[(Cr,P)O4]2}(H2O)n, where M2+ = Cu and Zn and 0.5 ≤ x + y ≤ 1, or, in the simplified form: (Pb,Cu,□)2{Pb[(Cr,P)O4]2}(H2O)n. The simplified formula of embreyite is similar in stoichiometry to vauquelinite and may explain the existence of the solid-solution series. The determination of the crystal structure of embreyite may also help to resolve the crystal chemical nature of cassedanneite. The XRD pattern of cassedanneite contains a distinct reflection with d = 13.9 Å, forbidden for the embreyite unit cell. This feature may indicate the doubling of the c unit-cell parameter of cassedanneite in comparison with embreyite. We assume that cassedanneite has structural similarity to embreyite with, presumably, a disordered distribution of Cr and V.
Associate Editor: Juraj Majzlan