No CrossRef data available.
Article contents
Fluoro-tremolite from the Limecrest-Southdown quarry, Sparta, New Jersey, USA: crystal chemistry of a newly approved end-member of the amphibole supergroup
Published online by Cambridge University Press: 28 February 2018
Abstract
During systematic characterization of amphiboles that still lack a complete mineral description, fluoro-tremolite was identified in a specimen from the Limecrest-Southdown quarry, Sparta, New Jersey, USA, which was provided by the Franklin Mineral Museum. The ideal formula of fluoro-tremolite is A□ BCa2CMg5TSi8O22WF2 and the empirical formula derived for the holotype specimen, based on the results of electron-microprobe analysis and single-crystal structure refinement, is A(Na0.28K0.02)Σ0.30B(Ca1.99Na0.01)Σ2.00C(Mg4.70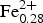 ${\rm Fe}_{{\rm 0}{\rm. 28}}^{{\rm 2 +}} $Zn0.01
${\rm Fe}_{{\rm 0}{\rm. 28}}^{{\rm 2 +}} $Zn0.01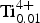 ${\rm Ti}_{{\rm 0}{\rm. 01}}^{{\rm 4 +}} $)Σ5.00T(Si7.68Al0.32)Σ8.00O22W(F1.16OH0.84)Σ2.00. The unit-cell dimensions in space group C2/m are a = 9.846(2), b = 18.050(3), c = 5.2769(14) Å, β = 104.80(2)° and V = 906.7 (3) Å3 and Z = 2; the a:b:c ratio is 0.545:1:0.292. Fluoro-tremolite is biaxial (+), with α = 1.5987(5), β = 1.6102(5), γ = 1.6257(5), 2V(meas.) = 85(1)o and 2V(calc.) = 82o. The strongest ten reflections in the powder X-ray pattern [d values (in Å), I, (hkl)] are: 2.706, 100, (151); 3.126, 67, (310); 2.531, 59, (
${\rm Ti}_{{\rm 0}{\rm. 01}}^{{\rm 4 +}} $)Σ5.00T(Si7.68Al0.32)Σ8.00O22W(F1.16OH0.84)Σ2.00. The unit-cell dimensions in space group C2/m are a = 9.846(2), b = 18.050(3), c = 5.2769(14) Å, β = 104.80(2)° and V = 906.7 (3) Å3 and Z = 2; the a:b:c ratio is 0.545:1:0.292. Fluoro-tremolite is biaxial (+), with α = 1.5987(5), β = 1.6102(5), γ = 1.6257(5), 2V(meas.) = 85(1)o and 2V(calc.) = 82o. The strongest ten reflections in the powder X-ray pattern [d values (in Å), I, (hkl)] are: 2.706, 100, (151); 3.126, 67, (310); 2.531, 59, (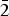 $\bar 2$02); 3.381, 57, (131); 2.940, 43, (
$\bar 2$02); 3.381, 57, (131); 2.940, 43, (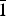 $\bar 1$51, 221); 3.276, 37, (240); 2.337, 36, (
$\bar 1$51, 221); 3.276, 37, (240); 2.337, 36, (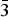 $\bar 3$51); 2.592, 35, (061); 2.731, 34, (
$\bar 3$51); 2.592, 35, (061); 2.731, 34, (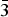 $\bar 3$31); 2.163, 34, (261). Both the mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2016–018); the holotype has been deposited at the Franklin Mineral Museum (32 Evans Street, Franklin, 07416 New Jersey, US), under the catalogue number 7710.
$\bar 3$31); 2.163, 34, (261). Both the mineral and the mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2016–018); the holotype has been deposited at the Franklin Mineral Museum (32 Evans Street, Franklin, 07416 New Jersey, US), under the catalogue number 7710.
Comparison with new data on tremolite and synthetic fluoro-tremolite provides a more sound crystal-chemical model of the end-member compositions and their solid-solution.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Sergey Krivovichev


