Published online by Cambridge University Press: 05 July 2018
Paratacamite-(Mg) (IMA 2013-014), Cu3(Mg, Cu)Cl2(OH)6, is the new Mg-analogue of paratacamite. It was found near the village of Cuya in the Camarones Valley, Arica Province, Chile. The mineral is a supergene secondary phase occurring in association with anhydrite, atacamite, chalcopyrite, copiapite, dolomite, epsomite, haydeeite, hematite, magnesite and quartz. Paratacamite-(Mg) crystals are rhombs and thick to thin prisms up to 0.3 mm in size exhibiting the forms {201} and {001}. Twinning by reflection on {10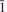 } is common. The mineral is transparent with a vitreous lustre, with medium to deep-green colour and light-green streak. Mohs hardness is 3–3½, the tenacity is brittle and the fracture is conchoidal. Paratacamite-(Mg) has one perfect cleavage on {201}. The measured and calculated densities are 3.50(2) and 3.551 g cm–3, respectively. The mineral is optically uniaxial (–) with ε = 1.785(5) and ω > 1.8 and slight pleochroism: O (bluish green) > E (green). Electron-microprobe analyses provided the empirical formula Cu3(Mg0.60Cu0.38Ni0.01Mn0.01)Cl2(OH)6. The mineral is easily soluble in dilute HCl. Paratacamite-(Mg) is trigonal, R
} is common. The mineral is transparent with a vitreous lustre, with medium to deep-green colour and light-green streak. Mohs hardness is 3–3½, the tenacity is brittle and the fracture is conchoidal. Paratacamite-(Mg) has one perfect cleavage on {201}. The measured and calculated densities are 3.50(2) and 3.551 g cm–3, respectively. The mineral is optically uniaxial (–) with ε = 1.785(5) and ω > 1.8 and slight pleochroism: O (bluish green) > E (green). Electron-microprobe analyses provided the empirical formula Cu3(Mg0.60Cu0.38Ni0.01Mn0.01)Cl2(OH)6. The mineral is easily soluble in dilute HCl. Paratacamite-(Mg) is trigonal, R , with cell parameters a = 13.689(1), c = 14.025(1) Å, V = 2275.8(3) Å3 and Z = 12. There is a pronounced sub-cell corresponding to a' ≈ ½a, c' ≈ c in space group R
, with cell parameters a = 13.689(1), c = 14.025(1) Å, V = 2275.8(3) Å3 and Z = 12. There is a pronounced sub-cell corresponding to a' ≈ ½a, c' ≈ c in space group R m. The eight strongest lines in the X-ray powder diffraction pattern are [dobs Å(I)(hkl)]: 5.469(87)(021), 4.686(26)(003), 2.904(34)(401), 2.762(100)(22
m. The eight strongest lines in the X-ray powder diffraction pattern are [dobs Å(I)(hkl)]: 5.469(87)(021), 4.686(26)(003), 2.904(34)(401), 2.762(100)(22 ,042), 2.265(81)(404), 1.819(26)(603), 1.710 (34)(440) and 1.380(19)(446). The structure was refined to R1 = 0.039 for 480 Fo > 4σF reflections. Refinement using interlayer Mg-Cu site scattering factors indicated that Mg is distributed statistically between both interlayer octahedra M1O6 and M2O6. A comparison of the distortions associated with M1O6 and M2O6 octahedra suggest that the sample is near the upper compositional limit for stability of the R
,042), 2.265(81)(404), 1.819(26)(603), 1.710 (34)(440) and 1.380(19)(446). The structure was refined to R1 = 0.039 for 480 Fo > 4σF reflections. Refinement using interlayer Mg-Cu site scattering factors indicated that Mg is distributed statistically between both interlayer octahedra M1O6 and M2O6. A comparison of the distortions associated with M1O6 and M2O6 octahedra suggest that the sample is near the upper compositional limit for stability of the R phase.
phase.