Introduction
Phenolic compounds or polyphenols are a complex group of phytochemicals possessing several hydroxyl groups on aromatic rings. They are widely distributed throughout the plant kingdom and thus form an integral part of the human diet(Reference Lindsay1, Reference Manach, Scalbert and Morand2). Several thousand molecules belong to this group and several hundreds can be found in edible plants. Polyphenols are secondary metabolites of plants thought to play a role in the protection against UV radiation and environmental pathogens(Reference Bravo3). They are commonly found in nature as glycosides and not in their free (aglycone) form. Glucose is the most common sugar residue, but galactose, rhamnose, xylose and arabinose are also found quite often(Reference Bravo3). Polyphenols can be classified into different groups according to the number of phenolic groups that they contain or the structural elements that bind the rings to one another. According to their structural features, polyphenols can be divided into at least ten classes (Table 1), the biological effects of phenolic acids, stilbenes and flavonoids being the most studied(Reference Manach, Scalbert and Morand2).
Table 1 Main polyphenol classes and representation of basic structures
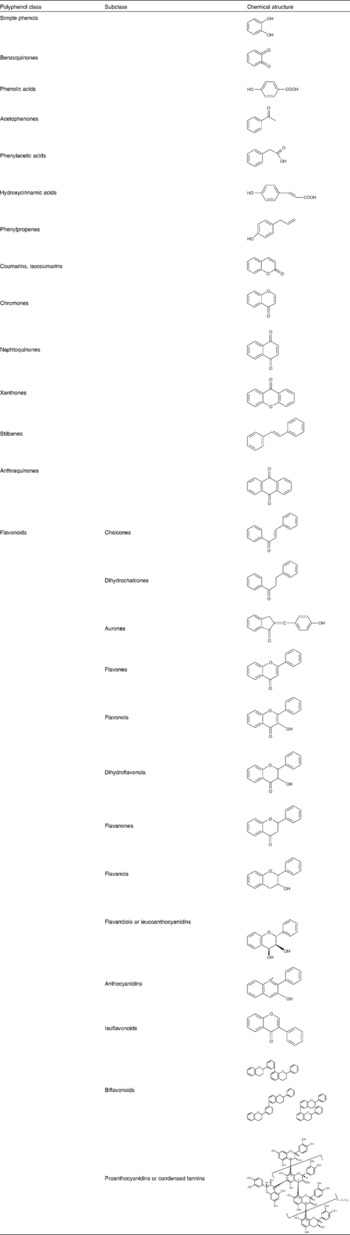
There are two major groups of phenolic acids: the derivatives of benzoic acid and the derivatives of cinnamic acid. Hydroxybenzoic acids include gallic acid which is abundant in tea but is not commonly found in its free form in plants eaten by humans. Rather, it is a component of complex structures such as hydrolysable tannins. Tannic acid is an example of gallic acid complexation: several molecules of the acid complex with glucose. Hydroxycinnamic acids comprise p-coumaric, caffeic and ferulic acids and are far more abundant in fruits where they occur mostly in the glycosylated form(Reference Manach, Scalbert and Morand2). Stilbenes are only found in low quantities in the diet. Resveratrol is the most extensively studied representative of this group of polyphenols. It is present in wine both in the aglycone and glycoside form(Reference Manach, Scalbert and Morand2). Flavonoids constitute the most important single group of polyphenols and can be subdivided into thirteen classes, with more than 5000 compounds included (Table 1)(Reference Bravo3). Flavonols are the most ubiquitous in food, and examples include quercetin, kaempferol and myricetin. Flavanols are also abundant and include monomeric forms, or catechins, and polymeric forms, also called proanthocyanidins or tannins. The polymeric forms include procyanidins and prodelphinidins(Reference Bravo3). Anthocyanins are another important class of flavonoids and constitute the major water-soluble pigments found in plants. They occur mainly as glycosides and the aglycones (anthocyanidins) are usually very unstable. In addition to glycosylation, their reaction with aromatic or aliphatic acids or other flavonoids is also possible(Reference Bravo3).
In recent years, an increasing interest in studying polyphenols, which constitute the active substances found in many medicinal plants, has been observed. The chief reason for this is the recognition of their antioxidant properties, their great abundance in the human diet, and their probable role in the prevention of various diseases associated with oxidative stress, such as cancer and cardiovascular, neurodegenerative and inflammatory diseases(Reference German and Walzem4–Reference Morton, Abu-Amsha Caccetta and Puddey9). Noteworthy is the fact that both consumers and the food industry are also gaining interest in this subject(Reference Manach, Scalbert and Morand2–Reference Block, Patterson and Subar10). The great abundance of polyphenols in beverages such as wine, and especially in red wine, has been advocated to be responsible for the beneficial effect of red wine consumption on heart disease, cancer and inflammatory diseases(Reference Manach, Scalbert and Morand2, Reference German and Walzem4, Reference Sun, Simonyi and Sun6, Reference Block, Patterson and Subar10–Reference van de Wiel, van Golde and Hart12). Apart from red wine, other beverages such as tea and beer constitute important dietary sources of polyphenols(Reference Manach, Scalbert and Morand2, Reference Mukhtar and Ahmad13, Reference Yang, Maliakal and Meng14).
In addition to their antioxidant properties, polyphenols have several other specific biological actions that are as yet poorly understood. For instance, polyphenols modulate the activity of a wide range of enzymes and cell receptors(Reference Middleton, Kandaswami and Theoharides5, Reference Stoclet, Chataigneau and Ndiaye7), and they also interfere with the activity and expression of several cell membrane transporters. In this context, the aim of the present paper is to review the information concerning the putative influence of these compounds upon the intestinal and placental cell membrane transport of some organic molecules with important biological functions.
The transport of organic compounds across cell membranes is largely determined by the activity of membrane-bound transport systems. Indeed, both nutrients (for example, glucose and vitamins) and bioactive compounds (for example, catecholamines and other organic cations (OC) such as histamine and serotonin) must use membrane transporters in order to efficiently cross biological membranes. As a result, the absorption, distribution and elimination of these compounds, as well as the extent of their biological activity, are largely dependent on more or less specific cell membrane-located transporters. This is especially true for transport across epithelial cells, which form barriers separating different compartments in the body. So, in the present review, the effect of polyphenolic compounds across two important biological barriers, the intestine and the placenta, will be considered.
The primary function of the intestinal epithelium is to absorb small molecules that are produced from digestion of food. Additionally, the intestinal epithelium constitutes one of the major routes of entry of drugs into the blood circulation. For this reason, intestinal transporters present both at the luminal-facing apical membrane and at the serosal-facing basolateral membrane of enterocytes will play an important role in promoting or limiting the absorption of exogenous compounds. There is presently a large debate on the ability of certain food components to interfere with the absorption of nutrients and drugs, resulting in alterations of their biological effects(Reference Middleton, Kandaswami and Theoharides5, Reference Harris, Jang and Tsunoda15). Many of these food–food or food–drug interactions can be explained by changes in the cellular uptake or extrusion of molecules.
Studies on the interaction of polyphenols with the intestinal absorption of nutrients have been mainly performed by using the Caco-2 cell line or rat intestinal tissue. Caco-2 cells are one of the most widely used cell models to study intestinal epithelial transport(Reference Artursson16–Reference Lennernas, Nylander and Ungell18), as these colonic adenocarcinoma-derived cells present an enterocyte-like phenotype(Reference Delie and Rubas19), forming confluent monolayers of cells with functional properties of transporting epithelia(Reference Lennernas, Nylander and Ungell18, Reference Hidalgo, Raub and Borchardt20, Reference Yee21).
As to the placenta, it constitutes the sole link between the mother and the developing fetus and performs a variety of functions that are essential for the maintenance of pregnancy and normal fetal development. One of the major functions of the placenta is to mediate the transfer of nutrients from the mother to the fetus and eliminate metabolic waste products from the fetus. This function is mediated by transporters present both at the maternal-facing brush-border membrane and at the fetal-facing basal membrane of the syncytiotrophoblast, a polarised epithelium that constitutes the functional unit of the placenta. The activity of these transporters will largely determine the extent at which organic compounds will cross the placenta and enter the fetal blood circulation.
Because polyphenolic compounds are obtained from dietary sources, the intestine is obviously expected to constitute a primary target for these compounds. Indeed, a beneficial effect of polyphenolic compounds at the intestinal level has been well documented(Reference Gee and Johnson22–Reference Scalbert, Deprez and Mila24). Thus, a putative interference of polyphenolic compounds with the intestinal absorption of nutrients, drugs and other exogenous compounds has been investigated in recent years. Moreover, polyphenolic compounds are, to a greater or lesser extent, absorbed from the gut lumen into the blood circulation(Reference Manach, Scalbert and Morand2, Reference Manach, Williamson and Morand25–Reference Scalbert, Morand and Manach27), and so the placenta will be exposed to these compounds, namely through the ingestion of polyphenolic-rich foodstuffs or beverages, such as wine, tea, coffee, etc. So, this organ represents as well a target for the action of polyphenols, which could also interfere with the placental uptake of nutrients or other bioactive substances from maternal circulation, and this also has been the subject of some investigation in the last few years.
Effect of polyphenols on the transport of 1-methyl-4-phenylpyridinium
Currently, there is a fair amount of results implicating phytochemical compounds in the modulation of the intestinal transport of several different kinds of substrates, including OC(Reference Faria, Mateus and de Freitas28–Reference Faria, Pestana and Monteiro31). The interest in the study of OC uptake modulation comes from the fact that a large number of biologically relevant organic molecules possess net charges at physiological pH. These include several classes of drugs (antihistamines, antacids, anti-arrythmics, anti-hypertensives and anticholinergics), but also essential molecules such as vitamins (thiamin and riboflavin), amino acids and bioactive amines (catecholamines, serotonin and histamine)(Reference Zhang, Brett and Giacomini32).
1-Methyl-4-phenylpyridinium (MPP+) (Fig. 1), a positively charged molecule at physiological pH, is widely used as a model OC substrate in intestinal uptake studies, since it is not metabolised in vivo (Reference Irwin, DeLanney and Di Monte33, Reference Sayre34) and is efficiently taken up by the intestinal epithelium(Reference Martel, Calhau and Azevedo35, Reference Martel, Grundemann and Calhau36). MPP+ is efficiently absorbed by Caco-2 cells in the apical-to-basolateral direction, and comparison of the characteristics of 3H-MPP+ apical uptake by Caco-2 cells and by HEK293 cells stably transfected with either the OC transporter OCT1 or the OCT3 (also known as the extraneuronal monoamine transporter) led to the conclusion that this process is mediated by these two transporters belonging to the amphiphilic solute facilitator (ASF) family, both of which were shown to be expressed in Caco-2 cells(Reference Martel, Grundemann and Calhau36). However, other transporters such as the serotonin transporter (SERT) might also be involved.

Fig. 1 Chemical structure of 1-methyl-4-phenylpyridinium.
Effect of wine on the intestinal transport of 1-methyl-4-phenylpyridinium
The first report on the interactions of polyphenol sources with MPP+ uptake by Caco-2 cells tested the effect of intact red or white wine in direct contact with the cell monolayers(Reference Monteiro, Calhau and Martel30). As known, wine is produced by the fermentation of crushed grapes from the species Vitis vinifera, and the growing concentration of ethanol during the procedure facilitates the extraction of compounds from the seeds, skins and stems of the fruit(Reference German and Walzem4). This results in a complex solution where more than 500 different kinds of molecules coexist. Technological variations in the winemaking process allow the production of red wine and white wine from the same grapes. For the latter, grape solids are removed earlier and fermentation goes on only in the presence of the juice. This has great impact on wine composition, mainly on the amount and type of polyphenols present in the final product. Red wine is composed of several different kinds of polyphenols: phenolic acids, stilbenes and flavonoids including flavonols, flavanols and anthocyanins(Reference German and Walzem4). Since grape seeds and skins are the parts of the fruit with the highest amount of polyphenols, red wine has about six times more polyphenols than white wine (about 1200 and 200 mg/l, respectively)(Reference German and Walzem4). So, while most polyphenols are usually present in both wines but in lower amounts in white wine (for example, resveratrol, catechins and procyanidins), some others are only found in red wine (for example, some phenolic acids, flavonols and anthocyanins)(Reference German and Walzem4).
Although the direct treatment of Caco-2 cells with the beverages may be debatable, it is recognised that some wine components may reach the intestinal epithelium intact. On the other hand, treatment with the whole beverages allows the detection of interactions between components of the complex matrix that is wine.
The study of Monteiro et al. (Reference Monteiro, Calhau and Martel30) revealed that red wine induced a concentration-dependent increase in 3H-MPP+ uptake into Caco-2 cells. The effect of red wine was partially abolished by the concomitant incubation of the cells with the OCT inhibitor decynium 22(Reference Martel, Grundemann and Calhau36). This suggests that there is an involvement of OCT in the effect of red wine upon 3H-MPP+ transport, but also that other routes of 3H-MPP+ entry into Caco-2 cells may also be affected by red wine. In contrast, white wine caused only a slight decrease in transport ability.
As the two wines tested had approximately the same amount of ethanol (12 %, v/v), it was concluded that the differences between their effects were most likely due to their non-alcoholic components. The fact that red wine has about six times more total polyphenols than white wine, and is about four times richer in high-molecular-weight polyphenols(Reference Monteiro, Calhau and Martel30), corroborated this assumption. Incubation of Caco-2 cells with alcohol-free wines or ethanol showed that the effect of alcohol-free red wine was significantly lower than that of red wine, and alcohol-free white wine showed a higher inhibitory potency on 3H-MPP+ uptake than white wine. Ethanol, on the other hand, inhibited 3H-MPP+ uptake in a concentration-dependent manner. So, the authors concluded that ethanol would exert some other kind of interaction with the remaining wine components, namely a facilitation of compound bioavailability or solubility that could facilitate their effect.
Effect of tea on the intestinal transport of 1-methyl-4-phenylpyridinium
Tea is a beverage prepared by the infusion of leaves of the plant Camellia sinensis. It is largely consumed worldwide, being increasingly related to beneficial health effects(Reference Trevisanato and Kim37). Drug interactions have been described after tea intake involving modulation of phase I and II biotransformation enzymes(Reference Harris, Jang and Tsunoda15), and the transport of molecules across cell membranes, namely through P-glycoprotein(Reference Jodoin, Demeule and Beliveau38).
Green tea and black tea are produced differently: for the first, leaves are dried only; however, to obtain the second, tea leaves are dried and then allowed to go through a chemical fermentation or oxidation process(Reference Trevisanato and Kim37). This reflects on the organoleptic properties of the two beverages and evidently on their chemical composition. Green tea has usually a higher amount of polyphenols (mainly the catechins epigallocatechin-3-gallate (EGCG), gallocatechin, epigallocatechin digallates, epicatechin digallates, 3-O-methyl epicatechin, epigallocatechin (EGC), catechin gallate and gallocatechin gallate) than black tea. Because of oxidation, black tea has a lower catechin level, since these are converted to theaflavins and thearubigins(Reference Yang, Maliakal and Meng14). Tea may also contain flavonoids from other groups, such as myricetin, quercetin and kaempferol and xanthines, especially theophylline and caffeine. Xanthines are more abundant in black tea, whereas green tea is especially rich in EGCG (30 % of dry weight, compared with only 9 % in black tea)(Reference Yang, Maliakal and Meng14).
When testing the effects of different concentrations of green and black tea on 3H-MPP+ transport by Caco-2 cells it was found that green tea (0·25 and 0·5 ml/ml) was able to increase by several fold the uptake of 3H-MPP+, in a concentration-dependent manner(Reference Monteiro, Calhau and Martel29). Moreover, black tea (0·5 ml/ml) also increased 3H-MPP+ uptake. However, its effect was significantly lower than that obtained with the same concentration of green tea. In the presence of the OCT inhibitors decynium 22 or corticosterone(Reference Martel, Grundemann and Calhau36), the stimulatory effect of tea upon 3H-MPP+ transport into Caco-2 monolayers was attenuated, suggesting that uptake of 3H-MPP+ may be mediated by both OCT and non-OCT routes.
Effect of isolated polyphenols on the intestinal transport of 1-methyl-4-phenylpyridinium
Grape seed-extracted procyanidins
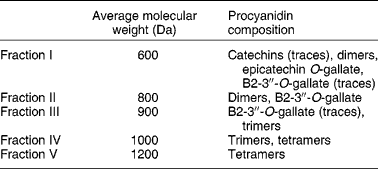
Procyanidins are a class of flavonoid compounds belonging to the flavan-3-ol subgroup that can be found in several food sources such as tea, apples and cocoa, being especially abundant in red wine. They are polymeric compounds formed of catechin and epicatechin monomers(Reference de Pascual-Teresa, Santos-Buelga and Rivas-Gonzalo39). Faria et al. (Reference Faria, Mateus and de Freitas28) studied the effect of a procyanidin extract isolated from grape seeds and of different size fractions of that extract (Table 2). During winemaking, these (and other) compounds are extracted from grape seeds and become soluble in wine(Reference Soleas, Diamandis and Goldberg11). The authors began to test the effect of a 60 min incubation period with procyanidins (6, 60 and 600 μg/ml) on 3H-MPP+ uptake by Caco-2 monolayers. In the lower concentration, fractions II and III inhibited 3H-MPP+ transport. These correspond to the low-molecular-weight flavan-3-ols. In the middle concentration, all fractions inhibited 3H-MPP+ uptake except fraction V; however, at the higher concentration, all fractions significantly increased 3H-MPP+ uptake, and the effect increased with increasing structural complexity of the fractions. Higher-molecular-weight procyanidins exist in higher concentration in red wine and in much lower amounts in white wine. Therefore, the effects reported by Faria et al. (Reference Faria, Mateus and de Freitas28) are well correlated with those described by Monteiro et al. (Reference Monteiro, Calhau and Martel30) showing opposing effects of red and white wine on 3H-MPP+ uptake and strengthen the procyanidin involvement in the effect of wine.
Table 2 Average molecular weights of procyanidins in grape seed fractions, determined by liquid secondary ion MS (adapted from Faria et al. (Reference Faria, Mateus and de Freitas28))
Experiments using the higher concentration of procyanidins, but different incubation periods (3 and 20 min), revealed that the effect of these compounds was also dependent on the time of contact with Caco-2 cells, stimulation of 3H-MPP+ uptake being more prominent for the longer incubation time (60 min). Since procyanidins, as good reducing and, therefore, antioxidant agents, are readily oxidisable due to their o-dihydroxyl groups(Reference Jovanovic, Steinken and Simic40), it was speculated that for the higher incubation time, oxidation of these compounds could change their interaction with or the state of the transporters involved in 3H-MPP+ uptake. This hypothesis was confirmed by examining the effect of cell exposure to the oxidised procyanidins. Indeed, oxidised procyanidins tested for 3 min increased 3H-MPP+ uptake to a level that was similar to the one found with non-oxidised procyanidins tested for 60 min. Furthermore, incubation for 60 min with oxidised procyanidins resulted in a significantly more pronounced stimulation of 3H-MPP+ entry into the cells. To confirm the cellular redox state involvement, both the effects of oxidant and reducing agents on the transport were tested(Reference D'Souza, Buckley and Buckley41). The results obtained with these compounds confirmed that intra- and extracellular oxidation status interferes with the transport. Three possible mechanisms which relate the redox state and OCT regulation have already been previously suggested: (a) changes in transporter affinity for the substrate; (b) changes in interaction of the transporter with the cytoskeleton, which will influence the number of membrane-located transporters; (c) regulation at the gene expression level(Reference Ciarimboli and Schlatter42). It is possible that the oxidation level of cysteine residues would alter the occurrence of phosphorylation and dephosphorylation reactions. This attractive hypothesis may indeed constitute the mechanism underlying the effects of procyanidins, because intestinal OC transport has been shown to be regulated by phosphorylation and dephosphorylation mechanisms(Reference Martel, Keating and Calhau43–Reference Calhau, Martel and Soares-da-Silva47), and polyphenols are known regulators of intracellular kinases and phosphatases(Reference Middleton, Kandaswami and Theoharides5).
Anthocyanins and derivative pigments
Anthocyanins are present in flowers, fruits and other vegetables, being an important group of plant pigments(Reference Manach, Scalbert and Morand2, Reference Bravo3). They exist in high amounts in blueberries (Vaccinium myrtillus), having been related to their health-promoting properties(Reference Santos-Buelga and Scalbert48).
Although growing scientific evidence of anthocyanin bioavailability is being gathered(Reference Manach, Williamson and Morand25), few studies have been devoted to the investigation of their possible interference with the absorption of other substrates at the intestinal level. Anthocyanins are glycosides, with the sugar moiety most commonly associated with the 3-position on the C-ring or the 5, 7-position on the A-ring (Fig. 2(a)). Glucose, galactose, arabinose, rhamnose and xylose are the most usually found sugars and can be bound as mono-, di- or tri-saccharide forms. Although there are about seventeen aglycone forms, or anthocyanidins, the most abundant and ubiquitously distributed are delphinidin, petunidin, peonidin, pelargonidin and malvidin. Apart from these more abundant compounds, some anthocyanin derivatives, such as anthocyanin pyruvic acid adducts and vinylpyranoanthocyanin-catechins (portisins), have been identified in low amounts in wine (Fig. 2(b) and (c)). These are more stable than their precursor anthocyanins and display unusual colours (orange and blue), being under investigation for their possible application as natural food dyes. Faria et al. (Reference Faria, Oliveira and Neves49) isolated anthocyanins from blueberries and characterised the obtained extract (extract I). This was composed mainly of the anthocyanins delphinidin, cyanidin, malvidin, petunidin and peonidin glucosides. This extract was used to synthesise anthocyanin pyruvic acid adducts (extract II) by reaction of the extract with pyruvic acid and vinylpryranoanthocyanin-catechins (portisins, extract III) after the reaction of anthocyanin pyruvic acid with catechin and acetaldehyde. In a subsequent study(Reference Faria, Pestana and Monteiro31), the ability of both the original blueberry and the derivate extracts to influence 3H-MPP+ apical intestinal uptake was studied, by incubating Caco-2 cells with 100 μg/ml of each extract for 60 min. Of the three tested extracts, only extract II had a significant effect, decreasing 3H-MPP+ uptake, an effect which showed concentration dependency. As there are no reports on the ability of OCT to transport anthocyanins or their derivatives, the possibility of an allosteric regulation or interference with the regulation of these transporters should not be excluded. Since molecular weight increases from extract I to III, size was thought not to be relevant to explain why only extract II showed an effect. On the other hand, it was advanced that the presence of a carboxyl group on the D ring of extract II components might play an important role and this was further explored by testing the effect of phenolic acids on the transport (see below).

Fig. 2 General structure of blueberry (Vaccinium myrtillus) anthocyanidin (a), anthocyanidin–pyruvic acid adducts (b) and portisins (c) present in extracts I, II and III, respectively. R1 and R2, independently of each other, are H, OH or O-methyl. R3 is glucose, galactose or arabinose.
Phenolic acids
The effect of four phenolic acids on the intestinal transport of MPP+ was tested by Faria et al. (Reference Faria, Pestana and Monteiro31). p-Coumaric, caffeic, ferulic, gallic and tannic acids (Fig. 3) were tested at 250 μg/ml for 60 min. Whereas gallic acid had no effect on the transport, the other phenolic acids induced a decrease in cellular 3H-MPP+ uptake. The authors suggested that the presence of a vinylphenolic group, with a possible high electronic conjugation, was likely to play a role. They also advanced the hypothesis that the effects observed resulted from ionic interactions between 3H-MPP+ and the dissociated acid moieties. As opposed to the other phenolic acids, the structurally more complex compound, tannic acid, increased MPP+ uptake by Caco-2 cells. Because this effect more closely resembles the one obtained with more complex flavonoids, such as procyanidins, than the ones obtained in the presence of other phenolic acids and monomeric compounds, molecular size was suggested to contribute to the effects.

Fig. 3 Structures of some phenolic acids. OMe, O-methyl.
Other monomeric polyphenols
The effect of some monomeric polyphenols (quercetin, myricetin, catechin and resveratrol) on MPP+ uptake by Caco-2 cells has also been investigated. Resveratrol did not affect 3H-MPP+ uptake, whereas all other compounds decreased it, quercetin having the strongest effect(Reference Monteiro, Calhau and Martel30).
The green tea polyphenol EGCG (2 mm) significantly increased 3H-MPP+ uptake, leading to the hypothesis that differences between black and green tea could be due to their different amounts of EGCG (see above). Procyanidin dimers and trimers can also be found in both teas, although green tea has almost double of the amount found in black tea(Reference Auger, Al-Awwadi and Bornet50). When tested upon 3H-MPP+ transport, 600 μg/ml of a procyanidin mixture (containing B1, B2 and B3 dimers and C1 trimers found in tea) stimulated this uptake(Reference Monteiro, Calhau and Martel29).
Effect of polyphenols on the transport of thiamin
Thiamin is a complex water-soluble B vitamin (vitamin B1), required by animal cells as the precursor of thiamin pyrophosphate, the coenzyme of the indispensable carbohydrate enzyme transketolase and the dehydrogenase complexes for pyruvate, α-ketoglutarate and branched-chain keto acids. Man and other mammals cannot synthesise thiamin and thus must obtain this vitamin from exogenous sources via intestinal absorption. Thiamin is a common food supplement in Western food products.
The intestine plays a critical role in regulating body thiamin homeostasis and understanding the mechanism of intestinal thiamin absorption process is of significant nutritional importance. In fact, thiamin plasma concentration is regulated both by intestinal and renal mechanisms. Additionally, this vitamin is extremely important for a normal fetal growth. Thus, placental transport, and its modulation, represents a crucial step in fetal development and human biology. However, excess thiamin supplementation in common food products may contribute to the increased cancer rates of the Western world(Reference Boros51).
Chemically, thiamin is a hydrosoluble OC with a high molecular weight. At concentrations lower than 2 μm, thiamin is absorbed by the intestinal mucosa mainly through active transport, a carrier-mediated system that precedes intracellular phosphorylation and dephosphorylation of this vitamin(Reference Gastaldi, Casirola and Patrini52). At these low concentrations, entry at the luminal side occurs largely through exchange with H+ and very little through enzymic transphosphorylation to thiamin monophosphate (TMP), by intestinal alkaline phosphatase present in the apical membrane of the enterocyte. Cellular crossing is associated with intracellular enzymic phosphorylation to thiamin pyrophosphate (TPP) and dephosphorylation of TPP to TMP and thiamin. At higher concentrations of thiamin, simple passive diffusion prevails(Reference Said and Strum53).
The expression of the recently cloned thiamin transporter, ThTr1, is very high in skeletal muscle, heart and placenta and almost absent in the intestine, kidney and brain(Reference Dutta, Huang and Molero54). These results are inconsistent with those obtained from functional studies showing a significantly higher thiamin uptake in the intestine and kidney than in skeletal muscle(Reference Rindi and Laforenza55, Reference Said, Ortiz and Kumar56). This seems to suggest the involvement of other intestinal transporter(s). In agreement with this, results obtained by our group showed that apical uptake of thiamin into Caco-2 seems to involve not only ThTr1 and ThTr2, but also one or more members of the amphiphilic solute facilitator (ASF) family of transporters(Reference Lemos, Calhau and Martel57).
Effect of isolated polyphenols on the intestinal transport of thiamin
There is no evidence that phenolic compounds affect thiamin transport into Caco-2 cells(Reference Lemos, Calhau and Martel57). However, non-alcoholic beverages such as green and black tea, as well as alcohol-free beer and alcohol-free red and white wines, were able to inhibit thiamin transport into Caco-2 cells, when tested acutely. In these same cells, the effect of some alcoholic beverages was tested (red and white wines and lager and stout beer), and the results were very curious. Only lager and stout beers inhibited transport; red and white wines had no effect. In agreement with these results, the inhibitory effect of alcohol-free wines seems to be abolished when ethanol (in the same concentration as that found in intact wines) was added. So, the biological activity of phenolic compounds, as modulators of thiamin uptake, depends on the presence or absence of ethanol. Accordingly with this conclusion, a recent study showed a crucial contribution of ethanol to phenolic bioavailability and/or bioactivity(Reference Faria, Pestana and Azevedo58).
An important point relates to the effect of xanthohumol. Although xanthohumol had no effect upon thiamin uptake into Caco-2 cells, lager and stout beers (which are both rich in this compound) inhibited its uptake. Additionally, stout beer, a chalcone-enriched beer, was more potent than lager beer. So, it is possible that the phenolic compounds xanthohumol and isoxanthohumol can explain, at least in part, the inhibitory effect found with the beers, as it happens with placental thiamin uptake(Reference Keating, Lemos and Azevedo59). If this is true, then (as it happens with red wine) the original matrix is important for the biological activity of these compounds.
Part of the results obtained with phenolic compounds in Caco-2 cells(Reference Lemos, Calhau and Martel57) was also confirmed in the rat. In these experiments, rats consumed red wine for 21 d and, at the end of this period, jejunal thiamin transport was evaluated in Ussing chambers, which allowed measurement of the mucosal-to-serosal apparent permeability to [3H]thiamin(Reference Lemos, Azevedo and Martel60). The results showed that a chronic consumption of red wine had no effect on thiamin absorption. However, the acute exposure of the rat jejunum to red wine was able to inhibit thiamin absorption, a result apparently contradictory to those observed with Caco-2 cells(Reference Lemos, Calhau and Martel57). However, two important reasons might explain the discrepancies found: (i) different species were studied (rat v. a human cell line; Caco-2 cells); (ii) different parameters were analysed (intestinal absorption in the rat v. uptake in Caco-2 cells).
Effect of isolated polyphenols on the placental transport of thiamin
As already stated, thiamin is crucial during pregnancy for the normal growth and development of the fetus. As needs for this vitamin increase during pregnancy, the modulation of thiamin transport through trophoblast epithelia constitutes a key point. Thus, any influence on this transport has consequences for fetal development. Indeed, the association between alcoholic abuse and thiamin deficiency is well known(Reference Hoyumpa61, Reference Hoyumpa, Breen and Schenker62), and thus it is possible that deficiency of this vitamin during pregnancy contributes, at least in part, to the developmental abnormalities observed in the fetal alcohol syndrome.
Considering this hot point, Keating et al. (Reference Keating, Lemos and Azevedo59) studied the short- and long-term effects of several phenolic compounds on the apical uptake of [3H]thiamin by BeWo cells. These cells are a human choriocarcinoma cell line, commonly used, and well characterised, as a trophoblast cell model. In the short term, no effect of xanthohumol, isoxanthohumol, catechin, epicatechin, resveratrol, quercetin, myricetin, EGCG, rutin and chrysin was found. In the long term (48 h), treatment with xanthohumol or isoxanthohumol significantly decreased thiamin uptake by these cells, but other compounds had no effect. Moreover, the inhibitory effect of xanthohumol and isoxanthohumol was not related to changes in mRNA levels for the thiamin transporters ThTr1 and ThTr2, as evaluated by RT-PCR. Also, these compounds had no effect on human SERT mRNA levels. Human SERT was suggested to be involved in the uptake of thiamin by these cells(Reference Keating, Lemos and Azevedo59). So, these effects are most probably not related to changes at the transcriptional level.
Altogether, it was found that chalcones, a class of phenolics found especially in beer, chronically inhibit the transport of thiamin. However, isoflavones, present in soya and in several functional foods such as several beverages and yogurts, were not tested. Considering that human SERT seems to be involved in thiamin uptake by BeWo cells, and that soya affects SERT activity(Reference Keating, Lemos and Azevedo59), it would be interesting to determine the effect of isoflavones upon thiamin placental transport. Indeed, Ito et al. (Reference Ito, Haito and Furumoto63) described an effect of beverages such as St John's wort (which is rich in phenolic compounds) on SERT activity that explains, at least in part, its psychopharmacological effects.
In relation to the results described by Keating et al. (Reference Keating, Lemos and Azevedo59) with chalcones, the mechanisms involved in this inhibitory action remain unknown. However, as is known, SERT is inhibited by phosphorylation pathways(Reference Kramer, Poblete and Azmitia64), and several phenolic compounds interact with the activity of kinases and phosphatases(Reference Said, Ortiz and Kumar56, Reference Kumar, Yanagawa and Ortiz65). Thus, it is possible that some phenolic compounds could interfere with transport activities through an indirect effect upon, for instance, phosphorylation and dephosphorylation mechanisms.
Effect of polyphenols on the transport of folic acid
Folic acid (FA; pteroylglutamate) is the parent structure of a large family of B vitamin coenzymes known as folates, which include FA (oxidised form) and reduced folates. The one-carbon derivatives of this water-soluble vitamin function as coenzymes in reactions leading to the synthesis of purine and pyrimidine precursors of nucleic acids, the metabolism of certain amino acids (methionine) and the initiation of protein synthesis in the mitochondria(Reference Herbert, Shils, Olson, Shike and Ross66, Reference Lucock67). Folates are thus essential for normal cellular functions, growth and development, and an adequate supply of this vitamin is necessary for normal human health. Folate deficiency, which constitutes the most prevalent vitamin deficiency in the Western hemisphere, is associated with megaloblastic anaemia, increased risk of CVD, cancer, Down's syndrome, Alzheimer's disease and defects in neural tube closure in developing embryos(Reference Herbert, Shils, Olson, Shike and Ross66–Reference van der Put, van Straaten and Trijbels71).
Effect of polyphenol-rich drinks and isolated polyphenols on the intestinal transport of folic acid
Because man cannot synthesise FA, this vitamin must be obtained from exogenous sources through intestinal absorption. Therefore, the intestine plays a central role in controlling and regulating FA body homeostasis, and any impairment in FA intestinal absorption may induce a whole-body deficiency state.
Effect upon [3H]folic acid permeability across the rat jejunum
The study of Lemos et al. (Reference Lemos, Azevedo and Martel60) was the first to investigate the effect of red wine upon the intestinal absorption of 3H-FA, by testing its effect upon the rat jejunal mucosal-to-serosal apparent permeability to 3H-FA. Red wine was tested both chronically in vivo (21 d consumption) and acutely in vitro. Interestingly, the mucosal-to-serosal apparent permeability to 3H-FA across rat jejunum was not changed either by the chronic ingestion of red wine (containing 12 % ethanol, v/v) or by the in vitro acute exposure of the tissue to red wine (diluted 1:5). From this lack of either acute or chronic effect of red wine upon the jejunal absorption of 3H-FA, it seems that there is a lack of effect of polyphenolic compounds in relation to the jejunal absorption of 3H-FA in the rat. Interestingly enough, the effect of ethanol was also assessed in the study by Lemos et al. (Reference Lemos, Azevedo and Martel60), and this compound was also found to have no effect on the mucosal-to-serosal apparent permeability to 3H-FA.
In the study by Lemos et al. (Reference Lemos, Azevedo and Martel60), the effect of polyphenolic compounds was assessed in the context of an alcoholic drink. This might be especially interesting, because chronic alcoholism has long been known to cause deficiency of several nutrients, including the vitamin FA(Reference Herbert, Shils, Olson, Shike and Ross66, Reference Thomson72–Reference van den Berg, van der Gaag and Hendriks75), and one of the causes of FA deficiency observed in chronic alcoholism is thought to be a reduction in the intestinal absorption of this vitamin(Reference Halsted, Robles and Mezey76, Reference Halsted, Robles and Mezey77). However, before this study, the effect of alcoholism upon the intestinal absorption of FA was investigated by analysing the effect of ethanol ingestion alone, which was thought to mimic the effect of alcoholic beverages. Because many alcoholic drinks contain polyphenolic compounds, which are currently known to be biologically active, it is now known that the effects of ethanol cannot be used to extrapolate to the effect of alcoholic drinks. Moreover, the effects of polyphenols alone also cannot be extrapolated to the effect of the drinks, because the effect of a single food component can be modulated by other food components.
Effect upon [3H]folic acid apical uptake by Caco-2 cells
The effect of polyphenolic compounds and some polyphenol-rich drinks (red and white wine, beer, tea and orange juice) upon the intestinal uptake of FA was also investigated by analysing their effect upon 3H-FA or [3H]methotrexate (3H-MTX; an anti-folate) uptake by Caco-2 cells(Reference Lemos, Peters and Jansen78).
Interestingly enough, all the tested beers (lager, stout and alcohol-free beer), green tea, black tea and orange juice (0·25 and 0·5 ml/ml) significantly inhibited 3H-FA and 3H-MTX apical uptake by Caco-2 cells. Moreover, both red and white wine (0·25 and 0·5 ml/ml) also significantly inhibited the apical uptake of 3H-FA by Caco-2 cells, red wine being more potent than white wine, and the same degree of inhibition was obtained with the alcohol-free wines. On the other hand, ethanol in the same concentration as that present in the red and white wine did also reduce 3H-FA apical uptake, but much less potently(Reference Lemos, Peters and Jansen78).
Because (1) ethanol had a much more discrete effect than the alcoholic drinks (wines and beers) upon 3H-FA uptake, and (2) alcohol-free drinks (red and white wine and beer) had almost the same effect as wine and beer, other components of these beverages must play a role in their inhibitory effect upon 3H-FA uptake. So, polyphenolic compounds present in all these drinks appear to have an inhibitory effect upon the intestinal absorption of this vitamin. This was confirmed by analysing the effect of some polyphenolic compounds known to be present in wines, beers and/or teas, in these same cells(Reference Lemos, Peters and Jansen78). When tested acutely, myricetin, EGCG and isoxanthohumol concentration-dependently inhibited 3H-FA uptake (50 % inhibitory concentration (IC50) values of 13, 8 and 36 μm, respectively). Myricetin and EGCG also had a concentration-dependent inhibitory effect upon 3H-MTX uptake (IC50 values of 11 and 10 μm, respectively) (isoxanthohumol was not tested). Other polyphenolic compounds (xanthohumol, resveratrol, quercetin and kaempferol) were found to moderately (20–50 %) inhibit the uptake of 3H-FA and/or 3H-MTX, but only when tested in a high (100 μm) concentration. Moreover, a long-term (2 d) exposure of the cells to isoxanthohumol resulted in inhibition of 3H-FA uptake; the other polyphenols were devoid of effect. Importantly, none of these compounds had a cytotoxic effect at concentrations that modulated 3H-FA and 3H-MTX uptake.
So, these results showed that (1) some polyphenolic compounds were able to significantly inhibit 3H-FA and 3H-MTX uptake by Caco-2 cells, and that (2) these phenolic compounds, when tested for a long period, lose, at least in part, their ability to reduce the apical uptake of 3H-FA in Caco-2 cells. The decrease in the inhibitory effect of polyphenols by long-term exposure to these compounds might result from an increased expression of FA transporter(s), resulting from FA depletion caused by acute exposure to phenolic compounds. This increased expression of FA transporters could compensate for, in long-term treatments, the effect of phenolic compounds.
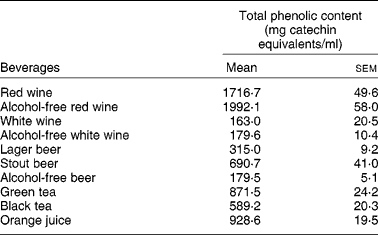
Interestingly enough, all the compounds that reduced the uptake of 3H-FA and 3H-MTX by Caco-2 cells (the stilbene resveratrol and the flavonols quercetin, myricetin, kaempferol) are very abundant in wines, where they are present in concentrations ranging from 1 μm to more than 300 μm(Reference German and Walzem4). So, it is possible that the phenolic compounds present in wines are, at least in part, responsible for the inhibitory effect of these beverages upon the intestinal uptake of 3H-FA. This hypothesis is supported by the fact that red wine, that has a much higher content of phenolic compounds than white wine (Table 3), also showed a more potent inhibitory effect than white wine.
Table 3 Total phenolic content of studied beverages (from Lemos et al. (Reference Lemos, Peters and Jansen78)) (Mean values (n 4) with their standard errors)
On the other hand, because beer constitutes the main dietary source of xanthohumol and other prenylflavonoids such as isoxanthohumol(Reference Stevens and Page79), and both xanthohumol and isoxanthohumol inhibited 3H-FA uptake, these compounds may be responsible for the inhibitory effect of beers on the intestinal uptake of this vitamin.
As to the inhibitory effect of green and black teas upon both 3H-FA and 3H-MTX by Caco-2 cells, the effect of both teas is possibly due to different polyphenols present in these beverages (see above). Thus, the effect of green tea might be explained by its high content of EGCG(Reference Mukhtar and Ahmad13), which was one of the most potent inhibitors of the uptake of both 3H-FA and 3H-MTX. Other phenolic compounds such as myricetin, quercetin and kaempferol can also be found in teas(Reference Yang, Maliakal and Meng14) and may also contribute to inhibition of 3H-FA and 3H-MTX uptake. In agreement with the results from Lemos et al. (Reference Lemos, Peters and Jansen78), green and black tea extracts, as well as two catechins contained in green tea (EGCG and epicatechin gallate (ECG)), were also found to inhibit FA uptake by Caco-2 cells(Reference Alemdaroglu, Wolffram and Boissel80).
The difference in the effect of red wine in relation to 3H-FA uptake in Caco-2 cells (inhibition)(Reference Lemos, Peters and Jansen78) and rat jejunum (no effect)(Reference Lemos, Azevedo and Martel60) can be explained by, at least, two important differences. First, the duration of the treatment with red wine is different in the two studies (a 48 h in vitro exposure of Caco-2 cells to red wine v. a 21 d in vivo ingestion of red wine). Second, the concentration of red wine in direct contact with the cells or tissue is also different (being higher in the experiments with Caco-2 cells). Finally, it should be noted that the process of digestion alters significantly the composition of red wine, and that this digestion was not assumed in the study by Lemos et al. (Reference Lemos, Peters and Jansen78).
As to the nature of the transport mechanism modulated by polyphenols, the observation that 3H-FA and 3H-MTX uptakes in Caco-2 cells were similarly modulated by most of the beverages and phenolic compounds suggests that these compounds share the same transport system in these cells. It is known that MTX and natural reduced folates are substrates of the reduced folate carrier (RFC)(Reference Jansen and Jackman81, Reference Matherly and Goldman82). However, it is not clear whether RFC is the major transporter involved in the intestinal transport of FA. Although many studies suggest a role for RFC in the transport of folates in intestinal cells(Reference Balamurugan and Said83–Reference Wang, Zhao and Russell87), recent evidence suggests the involvement of a proton-coupled FA transporter (PCFT). Interestingly enough, PCFT was recently identified as the solute carrier family 46, member 1 (SLC46A1)(Reference Qiu, Jansen and Sakaris88). So, the effect of polyphenols on FA uptake by Caco-2 may result from an inhibitory effect of these compounds upon RFC and/or PCFT.
In conclusion, the results obtained by Lemos et al. (Reference Lemos, Peters and Jansen78) suggest that the effect of several polyphenolic-rich beverages (red and white wine, beer, black and green tea and orange juice), significantly decreasing FA and MTX uptake, can be justified, at least in part, by the effect of their phenolic compounds. So, dietary habits, especially those related to the consumption of polyphenol-containing beverages or phenolic compounds, can modulate the intestinal uptake of both 3H-FA and 3H-MTX. Importantly, they may reduce the therapeutic efficacy of MTX in patients taking it by the oral route. Finally, these results suggest that, in human alcoholism, FA deficiency can result, at least partially, from a decrease in its intestinal absorption.
Effect of isolated polyphenols on the placental uptake of folic acid
Folate is also critically important for normal fetal development, as demonstrated by the well-established association between maternal FA deficiency and pregnancy complications such as pre-eclampsia(Reference Lucock67, Reference Scholl and Johnson89) and a higher incidence of fetal neural tube defects(Reference Lucock67). In line with this, periconceptional supplementation with FA is now widely accepted as a strategy for reducing the risk of neural tube defects(Reference Wald90, Reference Worthington-Roberts, Cohen, Cherry and Merkatz91).
Using the BeWo choriocarcinoma cell line, Keating et al. (Reference Keating, Lemos and Goncalves92) characterised the effect of several distinct polyphenolic compounds, present in alcoholic and non-alcoholic drinks, upon the placental uptake of FA. Both the short-term (26 min) and long-term (48 h) effect of several compounds (catechin, chrysin, epicatechin, EGCG, isoxanthohumol, myricetin, quercetin, resveratrol, rutin and xanthohumol) upon the uptake of 3H-FA by BeWo cells was determined(Reference Keating, Lemos and Goncalves92).
Interestingly enough, 3H-FA apical uptake by BeWo cells was found to be modulated by several dietary bioactive compounds. In the short term, epicatechin and isoxanthohumol inhibited 3H-FA uptake. Interestingly, the maximum effect was quantitatively similar for the two compounds (about 30 % inhibition). Isoxanthohumol seemed to act as a competitive inhibitor, whereas epicatechin caused an increase in both K m and V max. The authors hypothesised that epicatechin binds to an allosteric site of the transporter and induces an alteration in the conformation of the active site, thus reducing the affinity for the substrate (increasing K m) and that, simultaneously, this binding increases the transporter's capacity (increasing V max) for high concentrations of the substrate.
In the long term, 3H-FA apical uptake by BeWo cells was significantly increased by exposure to xanthohumol, quercetin and isoxanthohumol. At physiological pH, 3H-FA apical uptake by BeWo cells seems to involve both RFC and folate receptor (FR) α(Reference Keating, Lemos and Azevedo93). However, the increase in 3H-FA uptake caused by a long-term exposure of BeWo cells to the polyphenols was not accompanied by a change in RFC or FRα mRNA levels. So, the effect of the polyphenols does not seem to result from a modulation of the expression levels of these transporters. Instead, it may otherwise be a result of a direct interaction of the polyphenols with the transporter(s), with a consequent change in the activity of the latter(Reference Keating, Lemos and Goncalves92).
The cytotoxic effect as an explanation of the observed effect of polyphenolic compounds upon 3H-FA uptake was excluded. Moreover, in order to assess the specificity of the effect of polyphenolic compounds, the effect of these compounds upon the uptake of [14C]alanine was also assessed. Interestingly, none of these compounds had any significant effect upon uptake of this compound, except isoxanthohumol, which concentration-dependently and completely reduced uptake of this compound.
In summary, these results suggest a detrimental effect of short-term exposure to epicatechin and isoxanthohumol on placental FA absorption and, on the other hand, a beneficial effect of a long-term exposure to xanthohumol, isoxanthohumol and quercetin on FA absorption at the placental level(Reference Keating, Lemos and Goncalves92). Finally, these results also show that, because short- and long-term treatments with these dietary bioactive compounds did not produce parallel results, care should be taken when speculating about chronic effects from acute effects and vice versa(Reference Keating, Lemos and Goncalves92).
Effect of polyphenols on the transport of glucose
Effect of isolated polyphenols on the intestinal transport of glucose
Recent studies have suggested that ordinary portions of certain beverages rich in dietary phenols may result in an altered pattern of intestinal glucose uptake. However, this suggestion was based on the in vivo effect of beverages or plant extracts upon glycaemia or glucose tolerance(Reference Andrade-Cetto and Wiedenfeld94–Reference Matsumoto, Ishigaki and Ishigaki98), rather than on the direct effect of polyphenolic compounds upon the intestinal absorption of glucose. This latter subject was, however, also investigated in recent years, as shown next.
According to the ‘classical model of sugar absorption’, glucose is actively taken up into the enterocytes from the intestinal lumen by the high-affinity, Na+-dependent and phloridzin-sensitive Na+/glucose co-transporter 1 (SGLT1) located in the brush border and is then passively released from the enterocytes into the circulation via the Na+-independent GLUT2 present in the basolateral membrane (for reviews, see Drozdowski & Thompson(Reference Drozdowski and Thompson99), Wright et al. (Reference Wright, Martín and Turk100) and Kellett & Brot-Laroche(Reference Kellett and Brot-Laroche101)).
Several investigators suggested that some polyphenols decrease SGLT1-mediated glucose uptake. This conclusion was based on experiments using intestinal cells, brush-border membrane vesicles, or SGLT1-expressing Xenopus laevis oocytes(Reference Aoshima, Okita and Hossain102–Reference Wolffram, Block and Ader109).
Green tea flavonoids were found to inhibit the transport activity of SGLT1. While several flavonoids in green tea were active, this inhibitory activity was most pronounced for (+)-catechin and catechins having galloyl residues such as EGC and EGCG(Reference Hossain, Kato and Aoshima104, Reference Kobayashi, Suzuki and Satsu106, Reference Shimizu, Kobayashi and Suzuki110). According to Hossain et al. (Reference Hossain, Kato and Aoshima104), inhibition of SGLT1 by (+)-catechin, ECG and EGCG was independent of glucose concentration, suggesting a non-competitive inhibition mechanism. However, Kobayashi et al. (Reference Kobayashi, Suzuki and Satsu106) proposed a competitive inhibition mechanism for ECG in relation to SGLT1, although ECG itself is not transported via SGLT1. Because (+)-catechin inhibited glucose uptake weakly, it was concluded that probably it does not suppress glucose uptake in the small intestine under physiological conditions(Reference Hossain, Kato and Aoshima104, Reference Kobayashi, Suzuki and Satsu106). Tea catechin derivatives have also been reported to inhibit intestinal α-amylase or sucrase, which may be the main mechanism for the suppression of plasma glucose increase after a meal(Reference Matsui, Tanaka and Tamura111). Nevertheless, a crude extract of tea was shown to inhibit the intestinal absorption of glucose and Na+ in rats, although the relative contribution of the individual components involved was not determined(Reference Kreydiyyeh, Baydoun and Churukian112). Additionally, an instant tea preparation administered with a bolus of glucose was postulated to have similar effects in healthy human subjects(Reference Bryans, Judd and Ellis113). Thus, these catechin derivatives present in teas, wine or cocoa act possibly not only as antioxidants but also as inhibitors of glucose uptake in the small intestine, which may be helpful to diabetic patients.
Besides catechins, other polyphenolic compounds were also found to affect the intestinal absorption of glucose. Quercetin-3-O-glucoside inhibited SGLT1, apparently in a competitive manner(Reference Cermak, Landgraf and Wolffram103, Reference Wolffram, Block and Ader109, Reference Ader, Block and Pietzsch114). Quercetin-4-O-glucoside also inhibited SGLT1, but quercetin-3-O-galactoside, quercetin-3-O-glucorhamnoside (rutin) and the aglycone quercetin were devoid of effect(Reference Cermak, Landgraf and Wolffram103, Reference Ader, Block and Pietzsch114). Glycosides of some other flavonoid classes, such as naringenin-7-O-glucoside, genistein-7-O-glucoside and cyanidin-3,5-O-diglucoside, were ineffective as well(Reference Cermak, Landgraf and Wolffram103). According to some authors, quercetin glucosides such as quercetin-4-glucoside (the major dietary form of quercetin) are absorbed within the intestine by the active glucose transporter SGLT1, thus being an SGLT1 substrate(Reference Walgren, Lin and Kinne108, Reference Gee, DuPont and Rhodes115, Reference Hollman, de Vries and van Leeuwen116). However, other studies have concluded that neither quercetin nor any of its glycosylated derivatives are transported by SGLT1(Reference Kottra and Daniel117).
Emerging evidence indicates that besides SGLT1, there is also an involvement of the facilitated glucose transporter GLUT2 in the intestinal absorption of glucose(Reference Kellett and Brot-Laroche101, Reference Keating, Goncalves and Lemos118, Reference Kellett and Helliwell119). It is interesting to note that recent animal studies have suggested that luminal-facing GLUT2 is responsible for a large proportion of glucose uptake from the lumen of the small intestine. Being a major pathway of sugar absorption, GLUT2 is therefore an attractive target of potential agents(Reference Kellett and Brot-Laroche101, Reference Keating, Goncalves and Lemos118, Reference Kellett and Helliwell119).
Interestingly enough, some polyphenols were also found to interact with this transporter. Quercetin, quercetin-3-O-glucoside, ECG, fisetin, myricetin and gossypin decreased GLUT2-mediated Na+-independent diffusive uptake of glucose(Reference Cermak, Landgraf and Wolffram103, Reference Kwon, Eck and Chen120, Reference Chen, Hsu and Huang121). In relation to quercetin, its effect upon glucose uptake seems to be GLUT2-specific, because it does not interact at all with either SGLT1 or GLUT5(Reference Kwon, Eck and Chen120, Reference Song, Kwon and Chen122), although it does not appear to be a GLUT2 substrate(Reference Kwon, Eck and Chen120). In agreement with this observation, Song et al. (Reference Song, Kwon and Chen122) also reported that quercetin was a potent non-competitive inhibitor of GLUT2 expressed in Xenopus oocytes (K i of 23 μm). In contrast, quercetin-3-O-glucoside and ECG seem to be competitive inhibitors of GLUT2-mediated glucose transport(Reference Chen, Hsu and Huang121). Importantly, when diabetic rats were administered glucose together with quercetin, hyperglycaemia was significantly decreased compared with administration of glucose alone(Reference Song, Kwon and Chen122). Because the flavonoid quercetin, a food component, might act as a potent luminal inhibitor of sugar absorption independent of its own transport, flavonols show promise as new pharmacological agents in the obesity and diabetes epidemic(Reference Kwon, Eck and Chen120). Finally, exposure of Caco-2 cells for 48 h to some anthocyanins results in an increase in their own transport and in GLUT2 expression(Reference Faria, Pestana and Azevedo58), pointing to the possibility that these compounds are transported via GLUT2.
Some polyphenolic compounds have also been shown to be transported by other mechanisms, at the intestinal level. Indeed, quercetin-4-glucoside was found to be removed from Caco-2 cells by the apically expressed multidrug resistance-associated protein (MRP2), this process being able to decrease the net intestinal absorption of this compound(Reference Walgren, Karnaky and Lindenmayer107, Reference Walgren, Lin and Kinne108).
In a recent study, the effect of different classes of dietary polyphenols upon the intestinal uptake of glucose was investigated using Caco-2 cells(Reference Johnston, Sharp and Clifford105). Glucose uptake into cells under Na+-dependent conditions was inhibited by non-glycosylated polyphenols ((+)-catechin, ( − )-epicatechin, EGCG, EGC and ECG) whereas aglycones (quercetin, apigenin and myricetin), glycosides and phenolic acids (caffeic, ferulic and chlorogenic acids) were without effect. Under Na+-free conditions, aglycones (quercetin, apigenin and myricetin) and non-glycosylated polyphenols (EGCG, EGC and ECG) inhibited glucose uptake whereas glycosides and phenolic acids were ineffective. These data suggest that aglycones inhibit GLUT2-mediated uptake and that the non-glycosylated dietary polyphenols EGCG, ECG and EGC are effective against both GLUT2 and SGLT1.
The lack of effect of dietary glycosides (such as naringin, rutin and arbutin) upon both Na+-independent (i.e. GLUT-mediated) and Na+-dependent (i.e. SGLT1-mediated) uptake of glucose by Caco-2 cells(Reference Johnston, Sharp and Clifford105) is in agreement with previous results(Reference Ader, Block and Pietzsch114). However, the glycoside arbutin is transported by SGLT1 in hamster tissue(Reference Alvarado and Crane123) and in Xenopus oocytes(Reference Lostao, Hirayama and Loo124), and its consumption has been associated with ‘arbutin diabetes’(Reference Michel125). The lack of effect of the phenolic acids on glucose uptake under either Na+-dependent or Na+-free conditions(Reference Johnston, Sharp and Clifford105) is in contrast with the study by Welsch et al. (Reference Welsch, Lachance and Wasserman126) showing that caffeic, ferulic and chlorogenic acids caused an inhibition of Na+-dependent glucose uptake by rat brush-border membrane vesicles. The discrepancy in these observations may be related to the differences in the ratio of test substance to substrate(Reference Johnston, Sharp and Clifford105). Still according to these authors, the in vivo anti-hyperglycaemic effect of caffeic acid or chlorogenic acid extracts(Reference Andrade-Cetto and Wiedenfeld94, Reference Hsu, Chen and Cheng95) is most probably the result of a direct action on peripheral tissues rather than the result of a blockade of glucose uptake across the intestinal brush-border membrane(Reference Johnston, Sharp and Clifford105). Finally, the results of Johnston et al. (Reference Johnston, Sharp and Clifford105) concerning the effect of the non-glycosylated polyphenols are in perfect agreement with previous studies, either in vivo or in vitro (see above).
Still according to Johnston et al. (Reference Johnston, Sharp and Clifford105), the effects of EGCG, ECG and EGC are likely to be the result of steric hindrance caused by incorporation into the membrane with subsequent disruption of the surrounding lipid bilayer, as shown previously by Hossain et al. (Reference Hossain, Kato and Aoshima104) using transfected Xenopus oocytes as an expression vector.
In conclusion, recent in vitro evidence suggests that there is the potential for a variety of classes of dietary polyphenols to affect intestinal glucose transport in vivo mediated by both SGLT1 and GLUT2 simultaneously. Furthermore, these data suggest that foods and unsweetened beverages rich in these dietary polyphenols might provide a convenient dietary mechanism for regulating the rate of intestinal sugar absorption, an important factor in the management of diabetes, and in the long term might offer some protection against development of the metabolic syndrome or type 2 diabetes.
Effect of isolated polyphenols on the placental transport of glucose
Glucose is essential for the developing fetus, serving as the primary source of energy for metabolism and growth of the feto–placental unit. Because the fetus cannot synthesise it in the amounts required for its optimal development, it must obtain glucose from the maternal circulation. So, the supply of glucose from maternal blood to fetal circulation represents a major determinant of fetal growth and development(Reference Battaglia and Meschia127, Reference Harding and Johnston128). Glucose supply to the fetus is mediated by members of the GLUT family of transporters(Reference Bissonnette, Black and Wickham129–Reference Johnson and Smith131), GLUT1 being the predominant glucose transporter expressed at the placental level(Reference Barros, Yudilevich and Jarvis132–Reference Takata, Kasahara and Kasahara134).
The effect of several dietary polyphenols upon the placental transport of glucose was recently studied(Reference Araújo, Gonçalves and Azevedo135–Reference Araújo, Gonçalves and Martel137). By using [3H]deoxy-d-glucose (3H-DG), a glucose analogue which is efficiently transported by GLUT family members, several polyphenolic compounds were found to affect the apical uptake of 3H-DG into BeWo cells. When tested in the short term (26 min), resveratrol, EGCG and xanthohumol reduced 3H-DG uptake. Moreover, chrysin and quercetin decreased 3H-DG uptake in a concentration-dependent manner, causing a maximal reduction in 3H-DG uptake to 43 and 79 % of control, respectively. On the other hand, rutin, catechin and epicatechin increased 3H-DG uptake. It was also found that both quercetin and xanthohumol seemed to act as non-competitive inhibitors of 3H-DG apical uptake, whereas EGCG decreased both the K m and V max values. The effect of some polyphenolic compounds in association was also tested. Interestingly enough, catechin and epicatechin together decreased the apical uptake of 3H-DG, whereas epicatechin and xanthohumol counterbalanced each one's isolated effect. When tested in a long-term exposure (48 h), rutin and myricetin increased the apical uptake of 3H-DG both isolated and in combination(Reference Araújo, Gonçalves and Azevedo135–Reference Araújo, Gonçalves and Martel137).
Conclusions
Epidemiological evidence suggests that the consumption of polyphenol-rich foods reduces the incidence of cancer, CHD and inflammation. Phenolic compounds, numerous and ubiquitous in the plant kingdom, are particularly abundant in health-promoting foods.
Important conclusions concerning polyphenolic effects on transport systems can be drawn:
(i) different classes of polyphenols affect the transport of several bioactive compounds on two important biological barriers – the intestinal epithelia and the placenta;
(ii) different compounds belonging to the same phenolic family often possess opposite effects upon transport of a given molecule;
(iii) the acute (short-term) and chronic (long-term) exposures to these dietary bioactive compounds do not produce parallel results and, therefore, care should be taken when extrapolating results;
(iv) the effect of polyphenolic compounds in combination may be very different from those expected when taking into account the effect of each of these compounds alone, and so care should be taken when speculating on the effect of a drink based on the effect of one component only;
(v) care should be taken in drawing conclusions for alcoholic beverages from results obtained with ethanol alone.
In the future, it will be necessary to examine whether uptake of OC, thiamin, FA and glucose in the small intestine and placenta is modified in vivo when foods such as teas, chocolate or wine, which contain these active polyphenols, are consumed. Also, additional experiments are necessary in order to clarify the effects of these compounds on transport systems at the blood–brain barrier, as several beneficial effects of polyphenols on neuronal functions, particularly on appetite control and cognition, have been described. Moreover, application of more advanced experimental models, such as recombinant cell lines and genetically engineered mice, would certainly help in finding more definitive answers on identifying the effects of polyphenols on transport mechanisms. Therefore, intensive research is needed before a beneficial effect of polyphenol supplementation can be predicted. It is also necessary to clarify, not only the dietary effect of polyphenols, but also their toxicity to cells or organs when they are supplied as a dietary supplement.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
F. M., R. M. and C. C. contributed equally in writing the paper.
There are no conflicts of interest for the authors.








