Introduction
Selenium (Se) is a chalcogen trace element that is essential for human health(Reference Reich and Hondal1). Over the last three decades, there has been considerable advancement in our understanding of the sources and biological functions of Se. An important outcome of this research is the understanding that the health effects of Se depend upon the species of Se ingested and their metabolism(Reference Dumont, Vanhaecke and Cornelis2–Reference Rayman4). This insight corresponds with the current trend in toxicological and public health research of determining the diverse health effects of various forms or species of several other elements found in the natural environment (for example, mercury and arsenic)(Reference Muñoz-Olivas and Cámara5,Reference Zahir, Rizwi and Haq6) . In Inuit Nunangat (the Inuit homelands of the Canadian Arctic comprised of Inuvialuit Settlement Region, Nunavut, Nunavik, and Nunatsiavut), the traditional diet of Inuit populations (comprised of ‘country foods’, as they are called locally) is exceptionally high in Se, largely due to the presence of selenoneine (SeN) – an organoselenium compound and Se isologue of ergothioneine – in marine foods, and particularly beluga skin, that serve important roles in food security, nutrition, and cultural integrity(Reference Lemire, Kwan and Laouan-Sidi7,Reference Achouba, Dumas and Ouellet8) . As a result, Inuit populations across Inuit Nunangat exhibit considerably higher blood Se concentrations than other reference populations in North America and Europe(Reference Achouba, Dumas and Ouellet9,Reference Little, Achouba and Dumas10) . There is a need for both individuals, who may wish to take responsibility for their own health, and government agencies, which often establish public nutrition programming and nutrition guidelines, to be attentive to SeN as it relates to Se dietary sufficiency, metabolism, and health implications. The objective of this article is to review the current evidence on Se as it pertains to Inuit populations in the Canadian Arctic and make recommendations for cohesive, evidence-based research priorities, risk assessment, and public health decision-making that considers the presence of SeN as a major selenium species in several key marine foods.
Human selenoproteins
The biological actions and proposed nutritional essentiality of Se occur largely through selenoproteins. Selenium metabolism of major forms of dietary Se and selenoprotein synthesis are well-documented(Reference Rayman3,Reference Combs11–Reference Ha, Alfulaij and Berry14) . Organic forms of selenium, including selenomethionine (SeMet) and selenocysteine (Sec), are the most abundant forms of dietary Se, while inorganic compounds (selenite and selenate) represent a minor proportion of dietary intake(Reference Rayman15). Following absorption, Se compounds are mostly transported to the liver, which is the principal site of Se metabolism(Reference Ha, Alfulaij and Berry14). Dietary SeMet can be trans-selenated to Sec but is primarily non-specifically incorporated into body proteins (such as blood albumin) or converted to methylselenol (CH3SeH) (Fig. 1), although the importance of the latter process in humans is not known(Reference Okuno, Kubota and Kuroda16). Surplus Se may accumulate as SeMet in blood albumin or may be converted to methylated metabolites for excretion in the breath or (more commonly) urine(Reference Rayman3). In the liver, most Se compounds are metabolised to hydrogen selenide (H2Se). Subsequently, Sec is phosphorylated, leading to the formation of monoselenophosphate, which is used for the production of unique transfer RNA, Sec tRNA[Ser]Sec, that provides Sec for selenoprotein synthesis. In the presence of a Sec insertion sequence (SECIS), the UGA codon (which is normally a stop codon) is recoded to specify the insertion of Sec(Reference Labunskyy, Hatfield and Gladyshev17). A SECIS-binding protein recruits Sec tRNA[Ser]Sec for ribosomal translation and incorporation of Sec into nascent polypeptides(Reference Labunskyy, Hatfield and Gladyshev17).
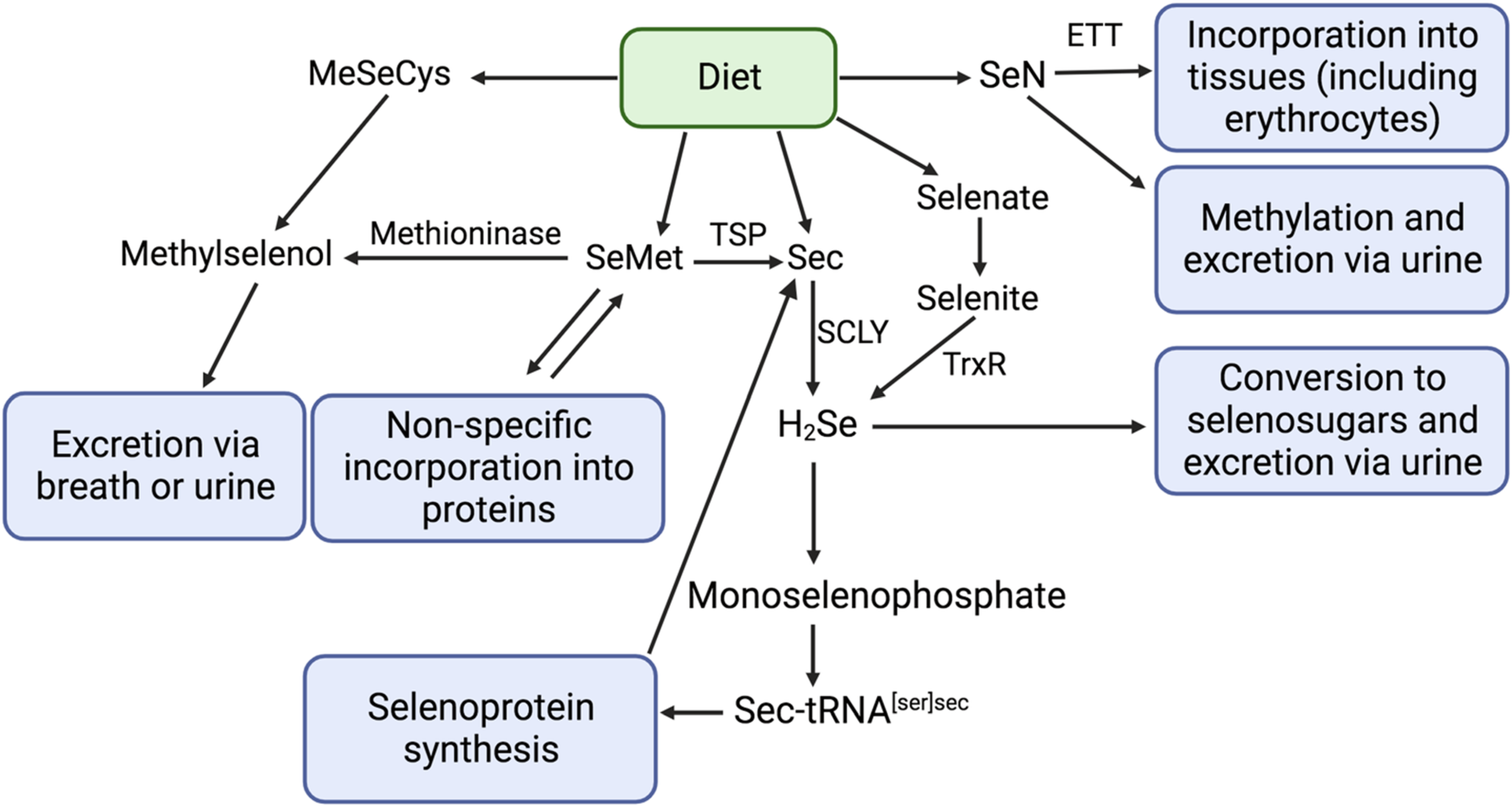
Fig. 1. Metabolism of Se food species, adapted from Combs (2001)(Reference Combs11), Kayrouz et al., (2022)(Reference Kayrouz, Huang and Hauser71), Rayman et al. (2008)(Reference Rayman3), Rayman (2012)(Reference Rayman15), and Yamashita et al. (2010)(Reference Yamashita and Yamashita65). ETT, ergothioneine transporter; SCLY, selenocysteine β-lyase; SeMet, selenomethionine; Sec, selenocysteine; H2Se, hydrogen selenide; CH3SeCys, Se-methyl-selenocysteine; SeN, selenoneine; CH3SeH, methyl selenol; TSP, transsulfuration pathway.
Approximately 25 selenoproteins have been identified thus far that play a functional role in a variety of physiological processes, including cell maintenance, oxidative homeostasis, thyroid hormone metabolism, brain activity, and immune response(Reference Labunskyy, Hatfield and Gladyshev17). For a summary of selenoproteins and their nomenclature and functions, please refer to Pitts and Hoffman (2018)(Reference Pitts and Hoffmann18). Optimum blood plasma Se levels are between 60 and 150 μg/L to maximize selenoprotein synthesis and activity(Reference Smith, Garg, Garg and Smith19,Reference Bleys, Navas-Acien and Guallar20) . It is commonly accepted that when Se intake is sufficient, plasma selenoprotein concentrations and activities plateau. Several researchers have therefore argued that plateau concentrations of plasma selenoproteins reflect functional Se sufficiency(Reference Duffield, Thomson and Hill21–Reference Yang, Zhu and Liu23). Consequently, total plasma Se concentrations and plasma selenoprotein (e.g., glutathione peroxidase 3 (GPX3) and selenoprotein P (SELENOP)) concentrations and activity levels are the most commonly used biomarkers for determining Se adequacy(Reference Thomson24).
Dietary reference values and safe upper limits
Although Se deficiency is rare, it is linked with reduced tissue concentrations and activity levels of selenoproteins and can contribute to Keshan disease (congestive cardiomyopathy caused by depletion of selenoprotein glutathione peroxidase, GPX), Kashin-Beck disease (atrophy and necrosis in cartilage tissue, possibly due to oxidative stress), hypothyroidism (due depletion of iodothyronine deodinases)(25), as well as increased risk of miscarriage and other reproductive and obstetric complications(Reference Al-Kunani, Knight and Haswell26–Reference Rayman, Wijnen and Vader29). Conversely, Se toxicity (selenosis) can occur with acute or chronic ingestion of excess Se. The most common adverse health impacts of selenosis are alopecia and nail brittleness and loss(Reference Yang, Wang and Zhou30), as well as gastrointestinal disturbances, skin rash, garlic breath odor, fatigue, irritability, and eventually nervous system abnormalities and paresthesia(Reference D’Oria, Apicella and De Luca31,Reference Yang, Yin and Zhou32) . Mechanisms of Se toxicity remain unconfirmed but selenosis likely occurs as a result of oxidative stress generation and consequent disruptions of cellular and mitochondrial function(Reference Mézes and Balogh33,Reference Spallholz34) . Biomonitoring equivalents associated with protection against selenium toxicity range from 400–480 μg/L in whole blood and 180–230 μg/L in plasma(Reference Hays, Macey and Nong35).
Over the past three decades, authoritative bodies have established dietary reference intakes (DRIs) for Se. In their 2001 assessment, the Institute of Medicine established the recommended dietary allowance (RDA) and tolerable upper intake limit (UL) at 55 μg Se/day and 400 μg Se/day respectively for individuals above 14 years of age(25). These values were subsequently adopted by several national regulatory authorities, including Health Canada(36) and the United States Department of Health and Human Services(37). This UL was reaffirmed in separate risk assessments conducted by the National Health and Medical Research Council of Australia and New Zealand(38) and the World Health Organization in coordination the Food and Agriculture Organization of the United Nations(39). Meanwhile, the Scientific Committee on Food (which provided the European Commission on scientific advice on food safety prior to the establishment of the European Food Safety Authority (EFSA)) established a UL of 300 μg Se/day(40) and the UK Expert Group on Vitamins and Minerals established a UL of 450 μg Se/day(41). While the methodology for these risk assessments varied slightly, all were based on a limited number of observational and experimental studies conducted in China(Reference Yang, Yin and Zhou32,Reference Yang and Zhou42,Reference Yang, Zhou and Yin43) , the US(Reference Longnecker, Taylor and Levander44), and New Zealand(Reference Duffield, Thomson and Hill21). Recently, following a request from the European Commission, the EFSA Panel of Nutrition, Novel Foods, and Food Allergens (NDA) undertook a systematic review to establish a scientific opinion on the UL for Se. Grounded primarily in data from the Selenium and Vitamin E Cancer Prevention Trial (SELECT), this panel recommended a UL of 255 μg Se/day based on a lowest-observed-adverse-effect-level of 330 μg Se/day and an uncertainty factor of 1·3(Reference Turck and Bohn13).
Case study: Selenoneine and Se status among Nunavimmiut
Inuit living in the Arctic have blood concentrations of Se that are among the highest in the world due to consumption of traditional country foods that are exceptionally replete in Se. (Table 1). Indeed, Inuit from Nunavik(Reference Achouba, Dumas and Ouellet9), Nunavut(Reference Laird, Goncharov and Chan45), and Greenland(Reference Hansen, Deutch and Pedersen46) have considerably higher whole blood Se concentrations than First Nations populations in southern Canada(47) and general populations in Canada(48), USA(Reference Jain and Choi49), and Europe.
Table 1. Whole blood Se concentrations in Inuit compared to other global populations
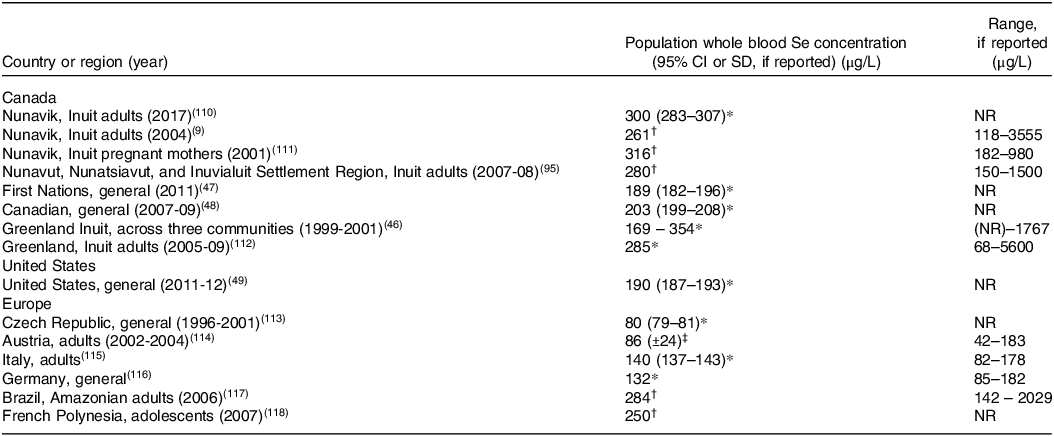
NR=not reported.
* Geometric mean.
† Median.
‡ Arithmetic mean.
Research involving Nunavimmiut (Inuit living in Nunavik, Québec) suggests a large portion of dietary Se is consumed as SeN, which is a major Se compound in RBCs in this population. Analyses on 881 blood samples collected during the Qanuippitaa? 2004 Nunavik Inuit Health Survey showed that SeN accounted for up to 92% of Se in red blood cells (geometric mean: 26%)(Reference Achouba, Dumas and Ouellet8). Findings from this study also suggest Se intake is approximately 214 µg/day (range: 10–1973 µg/day) in a representative sample of Nunavimmiut based on food frequency questionnaire data(Reference Rochette and Blanchet50) and using food Se concentrations derived from Navarro-Alarcon 2008(Reference Navarro-Alarcon and Cabrera-Vique51) and Lemire et al. 2015(Reference Lemire, Kwan and Laouan-Sidi7). Of all consumed foods, mattaaq (skin and underlying fat) derived from beluga whales, which is considered a delicacy by Inuit, is the richest source of total Se for Nunavimmiut(Reference Little, Achouba and Dumas10). Specifically, SeN accumulates in the skin layer and comprises the majority (median 54% in five samples) of Se found in beluga mattaaq (Reference Achouba, Dumas and Ouellet8). Consumption of beluga mattaaq is strongly correlated with RBC SeN concentrations among Nunavimmiut(Reference Little, Achouba and Dumas10). Lesser amounts of Se (including SeN) are also found in other traditional marine foods, including walrus(Reference Tremblay and Lemire52). This dietary Se profile differs from reference populations in southern Canada(Reference Thompson, Erdody and Smith53,Reference Hu and Chan54) , United States(Reference Engberg and Sylvester55–Reference Egan, Tao and Pennington57), Europe(Reference Mutanen58,Reference Waegeneers, Thiry and De Temmerman59) , New Zealand(Reference Thomson, Vannoort and Haslemore60), and Australia(61), who obtain Se almost exclusively through purchased meats, eggs, and cereals and other crops grown in Se-containing soil. Due to the accumulation of SeN in RBCs, Inuit exhibit a non-linear relationship between plasma and whole blood Se, in which plasma Se levels plateau around approximately 200 μg/L(Reference Achouba, Dumas and Ouellet9,Reference Hansen, Deutch and Pedersen46) . This contrasts with inland populations in Amazonian Brazil(Reference Lemire, Philibert and Fillion62), Malawi(Reference Stefanowicz, Talwar and O’Reilly63), and the United Kingdom(Reference Stranges, Laclaustra and Ji64), which exhibit a linear association between whole blood Se and plasma Se. Further, despite high whole blood Se, plasma Se and selenoproteins concentration among Inuit are in the normal ranges as reported elsewhere(Reference Achouba, Dumas and Ouellet9). Such findings therefore underscore that Se speciation in food plays a role in the Se species present, as well as their distribution in blood fractions, in consumers.
A closer look at selenoneine: A unique Se species from the marine environment
Selenoneine (2-selenyl-Nα,Nα,Nα-trimethyl-L-histidine or 3-(2-hydroseleno-1H-imidazol-5-yl)-2-(trimethylammonio) propanoate) is a selenoamino acid and Se-isologue to the sulfur-containing compound ergothioneine(Reference Yamashita and Yamashita65). SeN was identified in 2010 in the blood of bluefin tuna at concentrations in the range of 5–40 μg Se/g. Despite this, following more than a decade of subsequent research, SeN has also been reported in different biological matrices of marine animal origin, including beluga whale mattaaq (Reference Achouba, Dumas and Ouellet8), dolphins(Reference Pedrero Zayas, Ouerdane and Mounicou66), sea turtles(Reference Anan, Ishiwata and Suzuki67), various fishes (e.g., swordfish, Pacific mackerel, sardines, and tilapia)(Reference Yamashita, Yabu and Yamashita68), and seabirds(Reference El Hanafi, Pedrero and Ouerdane69), indicating trophic transfer through marine food webs. When found in animals, SeN is likely derived from the diet as only some fungi and bacteria synthesize ergothioneine and SeN(Reference Yamashita and Yamashita65,Reference Yamashita, Yamashita and Ando70,Reference Kayrouz, Huang and Hauser71) . Once consumed, SeN is transported across cell membranes by the ergothioneine transporter (ETT; formerly known as OCTN1), which is present in various tissues and organs(Reference Yamashita, Yabu and Yamashita68). In the bone marrow, uptake of SeN by maturing erythroid cells leads to SeN concentrating in red blood cells(Reference Gründemann, Hartmann and Flögel72).
Selenoneine and human health
Researchers have raised questions about potential health implications of SeN in animals, including humans(Reference Little, Achouba and Dumas10,Reference Yamashita, Yamashita and Suzuki73) . Such questions are particularly relevant to coastal populations that consume high amounts of marine foods, including Inuit living in northern Canada. As yet, however, relatively little is known about the chemistry and physiological functions of SeN.
SeN is one of several dietary Se species. The nutritional chemistry of Se is complex, and dietary Se compounds and their metabolites exhibit their own reactivity and biological activity. The metabolic pathways of the different forms of dietary Se and the relative abundance of Se metabolites are important to determine the overall health impacts of Se consumption (Fig. 1). Notably, as described above, hydrogen selenide (H2Se) plays a central role in Se metabolism; most dietary Se is transformed to H2Se before conversion to selenophosphate and incorporation into selenoproteins as Sec(Reference Berry, Banu and Chen74). However, SeN does not follow the H2Se metabolic pathway. Instead, SeN is distributed to organs and tissues via the ETT. In bone marrow, where the ETT is highly expressed, SeN is taken up by red blood cell precursors and incorporated into mature erythrocytes(Reference Yamashita, Yabu and Yamashita68,Reference Gründemann, Hartmann and Flögel72) . The physiological functions of SeN remain poorly elucidated. SeN has strong radical scavenging and antioxidant activity, and most researchers agree that this may be its primary function(Reference Alhasan, Nasim and Jacob12,Reference Yamashita, Yabu and Yamashita68,Reference Rohn, Kroepfl and Aschner75,Reference Tohfuku, Ando and Morishita76) . Indeed, it was shown to be more resistant to irreversible oxidative degradation compared to ergothioneine and engages in reversible oxidation and reduction reaction under conditions that irreversibly degrade ergothioneine(Reference Lim, Gründemann and Seebeck77). SeN has furthermore been shown to bind to myoglobin and hemoglobin to prevent auto-oxidation of iron(Reference Yamashita, Yabu and Yamashita68). SeN crosses the blood-brain barrier(Reference Drobyshev, Raschke and Glabonjat78) and a recent study showed that the SeN can accumulate in the brains of giant petrels(Reference El Hanafi, Pedrero and Ouerdane69). Authors suggest that SeN may play a role in the protection and function of the central nervous system. Additional implications on mammalian health have been noted; for example, animal model and in vitro studies have shown that SeN inhibits tyrosinase in melanoma cells and melanocytes (potentially by chelating copper at the active site of the enzyme)(Reference Seko, Imamura and Ishihara79), is protective against colorectal cancer in mice(Reference Masuda, Umemura and Yokozawa80), may attenuate hepatocellular injury and hepatic steatosis(Reference Miyata, Matsushita and Shindo81), and has ACE-inhibiting activity(Reference Seko, Imamura and Ishihara79).
Several metals, including lead, arsenic, cadmium, and Hg, form insoluble metal-selenide complexes in yeast, a reaction that may protect cells from both metal toxicity(Reference Kaur, Ponomarenko and Zhou82,Reference Ikemoto, Kunito and Tanaka83) and sodium selenide toxicity(Reference Dauplais, Lazard and Blanquet84). It is well recognized that Se can selectively bind with Hg and protect against MeHg toxicity, which is found in high concentrations at upper trophic levels of marine ecosystems(Reference Ikemoto, Kunito and Tanaka83). Recent experimental and epidemiological research provides evidence for the potential of Se to mitigate the cardiovascular and neurotoxic effects of MeHg exposure in humans(Reference Ayotte, Carrier and Ouellet85–Reference Branco, Canário and Lu91). Following the discovery of SeN, several researchers have suggested that it may play a role in the detoxification of MeHg, possibly through demethylation of MeHg leading to the formation of stable inorganic mercury selenide (Hg-Se) complexes(Reference Achouba, Dumas and Ouellet8,Reference Yamashita, Yamashita and Suzuki73,Reference Palmer and Parkin92) . Palmer and colleagues (2015) speculate that SeN promotes MeHg-induced proteolytic cleavage of Hg-C bonds, thereby demethylating MeHg prior to Hg-Se precipitation(Reference Palmer and Parkin92). Stable inorganic mercury selenide (Hg-Se) complexes are found to accumulate over time in the livers of marine birds and marine mammals, as well as in the brains of humans exposed to high levels of MeHg(Reference Ikemoto, Kunito and Tanaka83,Reference Korbas, O’Donoghue and Watson93,Reference Lailson-Brito, Cruz and Dorneles94) , indicating that demethylation mediated by SeN or other forms of Se occurs in vivo and may simultaneously reduce metal toxicity and functional availability of Se. Indeed, studies on zebrafish embryos showed reduced MeHg accumulation and toxicity in the presence of SeN(Reference Yamashita, Yamashita and Suzuki73). It is likely that such mechanisms have bearing on human health. Among Nunavummiut (Inuit living in Nunavut) and Nunavimmiut, high whole blood Se status (a large portion of which was likely SeN) exhibited a protective effect against the adverse cardiovascular health effects of high MeHg exposure(Reference Ayotte, Carrier and Ouellet85,Reference Hu, Eccles and Chan95) , suggesting that Se-mediated detoxification of MeHg may occur in humans. This is particularly relevant due to the elevated exposure of Inuit populations to MeHg through their traditional dietary staples, including marine mammals and predatory fish species(Reference Lemire, Kwan and Laouan-Sidi7).
Overall, current research paints an incomplete picture of the physiological functions and health implications of SeN. Despite growing interest in recent years, further biological assessment of SeN has been hampered by the absence of a commercial source(Reference Alhasan, Nasim and Jacob12). However, as mentioned, an important observation of the research to date is that SeN does not appear to contribute to the pool of H2Se for selenoprotein synthesis. Incubating cells with SeN causes no effect on GPX or SELENOP despite cells rapidly taking up the compound(Reference Drobyshev and Schwerdtle96). By contrast, incubation with reference selenium compounds selenite and selenomethionine induce increased activity of selenoproteins(Reference Allan, Lacourciere and Stadtman97). We can therefore conclude that SeN metabolism, biological function, nutritional essentiality, and toxicity differ from those of SeMet, Sec, and other better-understood Se species that are metabolized through the H2Se cycle. Furthermore, current evidence suggests that SeN is less toxic than other forms of Se(Reference Yamashita, Yamashita and Ando70). Drobyshev and colleagues (2023) demonstrated that SeN causes no toxic effects up to 100 µM concentration in hepatocytes and capillary endothelial cells(Reference Drobyshev and Schwerdtle96). Such findings add evidence to the suspicions of previous authors, including Yamashita et al., (2010), who posited that SeN has limited toxicity in their paper describing the discovery of SeN(Reference Little, Achouba and Dumas10,Reference Yamashita and Yamashita65) . Thus, individuals consuming a high percentage of Se as SeN may not experience the same detrimental health effects as populations consuming high amounts of SeMet, Sec, selenite, and selenate, despite high total Se intake and high whole blood total Se. Conversely, populations consuming the majority of their dietary Se as SeN may need to ensure they have other dietary sources of Se to ensure adequate selenoprotein synthesis and activity.
The flaws of current Se recommendations for Inuit populations living in Canada
Dietary Se guidelines and information sheets often refer to dietary reference intakes established by the Institute of Medicine, with the goal of preventing overt signs of deficiency and excess(36). Under Health Canada’s Chemicals Management Plan, which aims to assess and manage chemicals to “protect the health of Canadians and the environment”, the Government of Canada has published an assessment of Se and its compounds(98). As a part of this assessment, Health Canada prepared and distributed an overview of information on Se focused on North and Northern communities, which notes that “Se can be harmful to human health at levels above what the body needs to function” and “blood levels of selenium above the international guidance level (i.e. 480 microgram/L) have been measured in up to 28% of Inuit” (Health Canada, Information on Selenium in the North, 2018, personal communication). Notably, however, these assessments and communication contain no reference to SeN, which comprises one of the primary species of dietary Se among Nunavimmiut and likely all Inuit living in northern Canada.
The continued failure to disaggregate Se species in research, dietary guidelines, and communications about Se is problematic and may lead to unnecessary concern about selenosis among Inuit populations. This trend is reflected in the fact that RDAs and ULs apply to total Se intake, thereby overlooking dietary Se speciation and disregarding the varied functions of dietary Se compounds and metabolites(Reference Rayman3). The RDAs developed by the IOM are based on only two experimental studies – one conducted by Yang and colleagues (1987) in China(Reference Yang, Zhu and Liu23), and one conducted by Duffield and colleagues (1999) in New Zealand(Reference Duffield, Thomson and Hill21). These foundational studies have limited external validity due to their small sample sizes, interventions that comprised only one Se species (SeMet) or unquantified Se species, and a limited number of female, youth, and elderly participants. Such limitations minimize the generalizability of findings to other global populations, including Inuit in northern Canada, for whom SeMet is not the primary form of dietary Se. Further, studies that informed the development of RDAs used GPX activity as an indicator of Se sufficiency(Reference Duffield, Thomson and Hill21,Reference Yang, Zhu and Liu23) . A major limitation of this approach is that, despite their contributions to total dietary Se intake, SeN accumulates in RBCs and has little bearing on plasma Se or selenoprotein synthesis or activity, as stated earlier. Indeed, evidence suggests that Inuit populations exhibit normal levels of selenoproteins despite very high total Se intake and RBC Se status(Reference Achouba, Dumas and Ouellet9).
Similarly, ULs promoted by the IOM are based on two observational studies – one conducted by Yang and colleagues (1994) in Enshi, China(Reference Yang and Zhou42), and another conducted in western United States(Reference Longnecker, Taylor and Levander44). These studies were once again limited in their external validity due to small sample sizes and unspecified Se species and exposure routes, thereby limiting their relevance in determining ULs for Inuit populations. The recent systematic review and scientific opinion published by the EFSA NDA recommended lowering the UL from 300 to 255 μg Se/day. While this review recognized the existence of SeN in marine foods, their risk assessment failed to consider dietary Se speciation in establishing ULs.
Despite very high dietary intake of Se (often exceeding ULs promoted by the IOM and EFSA NDA) and whole blood Se concentration, Inuit populations in Nunavut(Reference Hu, Eccles and Chan95), Nunavik(Reference Achouba, Dumas and Ouellet9), and Greenland(Reference Hansen, Deutch and Pedersen46) exhibit little evidence of selenosis. Since marine food consumption has declined rapidly following colonial policies enforced by the Government of Canada (e.g., forced settlement and introduction of retail foods)(Reference Little, Hagar and Zivot99), it is reasonable to assume that SeN intake was considerably higher prior to colonial contact. Although data do not exist prior to 1992, there is no historical record of selenosis (or symptoms thereof) among Inuit. It is likely that dietary Se speciation accounts for variations in perceived tolerances of total Se intake between populations. For example, it has been shown that selenite ingestion leads to excess at much lower doses compared to SeMet(25), while SeN appears to be a non-toxic form of Se, as previously mentioned(Reference Yamashita, Yabu and Yamashita68). Overall, this research suggests that current DRIs and recommendations on Se are not relevant for Inuit populations, and future risk assessments and communications regarding Se exposure in northern Canada need to be cognizant of dietary intake of SeN in combination with other Se species.
Future directions for research and risk assessment incorporating evidence on SeN
There remain several gaps in our understanding of SeN. First, little is known regarding the natural synthesis and origins of SeN in the marine food chain. Ergothioneine, the sulfur analogue of SeN, is synthesized by bacteria and fungi but not plants or animals(Reference Jones, Doyle and Fitzpatrick100), and researchers have speculated that the same is true of SeN(Reference Kayrouz, Huang and Hauser71). Recently, Kayrouz et al. (2022) used a genome-mining strategy to identify a three-gene cluster that encodes a dedicated enzymatic pathway for producing selenoneine in bacteria, disproving prior theories that selenoneine is synthesized due to non-specific incorporation of Se during ergothioneine production(Reference Kayrouz, Huang and Hauser71). Since animals do not synthesize SeN, marine species exhibiting high concentrations of SeN (e.g., beluga whales and tuna) likely obtain SeN through dietary sources or through their microbiome(Reference Achouba, Dumas and Ouellet8), however additional research is necessary to identify and confirm natural sources of SeN. Furthermore, given the emerging nature of evidence on SeN, there is a need for research on SeN kinetics, metabolic pathways, biological functions, and health implications to appropriately assess the benefits and potential risks of SeN consumption. Such research must consider Se and SeN bioavailability and metabolism vis-à-vis consumption of metallic elements, including MeHg. It is also imperative that researchers, health practitioners, and public health agencies work together to identify and appropriately deploy relevant and appropriate biomarkers of Se status. In particular, whole blood Se concentration may be a poor measure of Se adequacy for selenoprotein function, considering SeN accumulates in red blood cells but does not serve as a Se reservoir for selenoprotein synthesis. Researchers should instead measure plasma Se and selenoproteins (e.g., SELENOP concentration and GPX activity) as biomarkers of Se functional sufficiency. Meanwhile, there is a need for more widespread measurement of SeN levels among humans to determine the concentrations and distribution of this compound across global populations. Recent advances in SeN analytical methods published by Achouba and colleagues (2023) should make this process more accessible, sensitive, specific, precise, and cost effective(Reference Achouba, Dumas and Ayotte101).
It is important to recognize the value of traditional country foods of marine origin, which are often high in Se, to the cultural integrity and food basket of Inuit populations. This recognition must permeate all research and public health messaging that occur with Inuit populations in northern Canada. Above all, country foods play an integral role in Inuit life by providing a spiritual connection to the land(Reference McGrath-Hanna, Greene and Tavernier102) and improving nutritional status(Reference Kuhnlein and Receveur103,Reference Johnson-Down and Egeland104) , food security(Reference Chan, Fediuk and Hamilton105), and mental health(Reference McGrath-Hanna, Greene and Tavernier102,Reference Lucas, Dewailly and Blanchet106) . Thus, it is important to recognize the dangers of endorsing and disseminating existing ULs for Se, as such actions may exacerbate current fears surrounding the consumption of country foods that have arisen due to zoonotic diseases (e.g., Giardia spp., Trichinella spp., Toxoplasma gondii, etc.) and environmental contaminants (e.g., MeHg and persistent organic pollutants, among others)(Reference Pufall, Jones and McEwen107,Reference Donaldson, Van Oostdam and Tikhonov108) . Given the significance of country foods to Inuit populations, we must be careful to not discourage country food consumption due to its importance for food security and nutrition(Reference Lemire, Kwan and Laouan-Sidi7). It is therefore crucial to provide the best evidence on Se and SeN to local public health practitioners and clinicians (including physicians and midwives) to help them promote country foods while minimizing the risk of exposure to harmful contaminants when designing and implementing public health education and clinical recommendations on environmental contaminants, Se, and other country food nutrients among Inuit populations.
While this case study has focused primarily on the Inuit populations, our arguments likely have broader relevance. SeN is found in high concentrations in many marine animals that serve as staple food sources for populations globally. Marine foods are especially crucial to the food security, nutrition, and cultural traditions of coastal populations, including coastal Indigenous populations(Reference Cisneros-Montemayor, Pauly and Weatherdon109). For example, SeN has been also identified as a major Se compound found in the blood of human populations consuming large amounts of marine foods in northern Japan(Reference Little, Achouba and Dumas10,Reference Yamashita, Yamashita and Ando70) . Although population-level analyses of blood SeN concentrations are extremely limited, we posit that SeN may comprise a large fraction of whole blood Se in coastal populations around the globe. As such, the evidence reviewed in this manuscript, and the arguments emerging therefrom, may be broadly applicable to coastal populations globally. There is a need for additional research on Se status, Se adequacy, and SeN sources and whole blood concentrations in understudied coastal populations. Following this, there is a need to incorporate such evidence into our existing body of research, DRIs, and public health guidance regarding Se to reflect the presence of SeN in foods and human populations. As a further complication, SeN and MeHg often occur in high concentrations in the same marine foods (e.g., Lemire et al. 2015(Reference Lemire, Kwan and Laouan-Sidi7)) and are highly correlated in human populations (e.g., Achouba et al. 2019(Reference Achouba, Dumas and Ouellet8)). Such evidence must comprise an important component of any risk assessment and public health strategy on Se.
Conclusion
In recent years, there have been substantial advancements in the study of different chemical forms of Se in food sources and tissues. The recent discovery of SeN, a selenoamino acid and Se-isologue to the sulfur-containing compound ergothioneine that accumulates in red blood cells, underscores the importance of Se speciation in research, risk assessment, and dietary reference intakes. In this article, we have argued a case to evaluate and reconsider the relevance of public health recommendations on Se with a special focus on Inuit in northern Canada, who consume a large portion of their dietary Se as SeN. Our arguments may have relevance for other populations who consume marine diets high in SeN. Since SeN does not appear to be as toxic as other dietary Se species and does not contribute to synthesis of selenoproteins, it is important to consider nuanced dietary and public health guidelines for Se that are responsive to emerging evidence. While selenoneine has limited relevance to Se metabolism involving synthesis of selenoproteins, there is a need for further research on the health implications of this compound, including its potential to serve as a strong dietary antioxidant and detoxifying agent for methylmercury.
Acknowledgements
We are grateful to all participants of research studies conducted in Nunavik and elsewhere in Inuit Nunangat over the past decades, including the 2004 Qanuippitaa? Inuit Health Survey, the 2007-08 International Polar Year Inuit Health Survey, and the 2017 Qanulirppitaa? Inuit Health Survey. We also acknowledge the contributions of Cole Heasley, who provided comments on previous drafts of the manuscript.
Financial support
This research was funded by a post-doctoral fellowship through the Canadian Institutes of Health Research, ArcticNet grant no. P74, and Crown-Indigenous Relations and Northern Affairs Canada Northern Contaminants Program grant no. H-12. Matthew Little is a Michael Smith Health Research BC Scholar. Mélanie Lemire is a member of Quebec Océan and also received a salary grant from the Fonds de recherche du Québec – Santé (FRQS): Junior 1 and 2 (2015-2023) and Senior (2023-2027). She is the titular of the Littoral Research Chair – the Sentinel North Partnership Research Chair in Ecosystem Approaches to Health (2019-2024), which is mainly funded by Sentinel North and by the Northern Contaminants Program (NCP) of the Crown-Indigenous Relations and Northern Affairs Canada.
Competing interests
The authors declare none.
Authorship
Matthew Little: Conceptualization, Data curation, Funding acquisition, Formal analysis, Writing – original draft, Writing – review & editing Adel Achouba: Conceptualization, Writing – review & editing Mélanie Lemire: Conceptualization, Data curation, Formal analysis, Writing – review & editing Pierre Ayotte: Conceptualization, Data curation, Formal analysis, Writing – review & editing. All authors read and approved the final manuscript.





