Introduction
Overweight and obesity are emerging problems all over the world – not only developed countries but also recently industrialised and developing countries are struggling under the burden of this epidemic(1). In Germany, one in five adults suffers from severe obesity, indicated by a BMI of 30 kg/m2 or higher(Reference Berghofer, Pischon and Reinhold2) and even 60 % of German adults are overweight (i.e. with a BMI of 25 kg/m2 or higher)(Reference Stevens, Singh and Lu3). Apart from limitations in everyday life such as impaired physical performance or dyspnoea caused by obesity, the incidence of associated disorders severely increases the more body weight is gained(Reference Alberti, Eckel and Grundy4–Reference Martin-Montalvo, Mercken and Mitchell6). In the past, chronic diseases like type 2 diabetes mellitus (T2DM), hypertension, CVD and cancer were mostly observed in the elderly. Nowadays, many young individuals are also constrained by these pathologies because of excess weight(Reference Ford, Giles and Dietz7). It is estimated that the worldwide mortality from chronic diseases will increase up to 66 % in 2030(1). For this reason, food and health agencies such as the USDA (United States Department of Health and Human Services and United States Department of Agriculture) recommend choosing foods high in vitamins and minerals but low (to moderate) in energy density in order to decrease the risk of such diet-related diseases. Indeed, by changing Western eating patterns, enhancing physical activity levels and avoiding tobacco use, the risk of CVD and T2DM as well as of cancer could be reduced by 80 and 40 %, respectively(1).
It has been hypothesised that ageing and its associated diseases may be the result of increasing amounts of altered nuclear and mitochondrial DNA, structurally aberrant proteins, and oxidised lipids that lead to structural and functional impairment of single cells and whole organisms(Reference Morselli, Galluzzi and Kepp8, Reference Toth and Tchernof9). This may impede dealing with and recovering from endogenous and environmental stress, thereby favouring the development of age-related chronic diseases(Reference Kim, Jung and Yu10, Reference Rae, Butler and Campisi11).
In the following, current literature on energy restriction (ER; also known as caloric restriction), the most promising method to counteract obesity and ageing, and on its underlying mechanisms such as anti-inflammatory and antioxidant effects is reviewed. Additionally, substances that have been discussed as potentially mimicking ER are also discussed.
Energy restriction and lifespan in model organisms
ER, a reduction in energy intake of 20 % (mild ER) to 50 % (severe ER)(Reference Merry, Kirk and Goyns12, Reference Minor, Smith and Sossong13) without a reduction in essential nutrients or malnutrition(Reference Yu14), was shown to reduce obesity and prevent premature onset of chronic ageing-associated diseases(Reference Weindruch15). Up to now ER is the only intervention which reliably increases lifespan in various model organisms(Reference Kim, Jung and Yu10).
In an early study in 1935, rats on a hypoenergetic diet showed increased lifespan(Reference McCay, Crowell and Maynard16). Since then several studies have verified these findings in model organisms extending from yeast(Reference Wu, Liu and Huang17) over invertebrate species like Caenorhabditis elegans (18) and Drosophila melanogaster Reference Min, Yamamoto and Buch(19) to rodents and primates(Reference Weindruch, Walford and Fligiel20–Reference Colman, Anderson and Johnson22). Conversely, there are reports that ER may also shorten lifespan in mammals(Reference McCay, Crowell and Maynard23) and a meta-analysis by Swindell(Reference Swindell24) concluded that the ER-induced lifespan increase in rodents depended on the genotype. Furthermore, it is questionable whether ad libitum-fed animals are the adequate controls for ER studies because ad libitum intake can lead to overweight and consequently shorten lifespan(Reference Sohal and Forster25). In contrast to an earlier study in rhesus monkeys at the Wisconsin National Primate Research Center(Reference Colman, Anderson and Johnson22), Mattison et al. (Reference Mattison, Roth and Beasley26) found, on the one hand, that ER did not affect the animals’ lifespan. On the other hand, the ER-fed monkeys showed a later onset of age-related diseases than the ad libitum-fed controls and therefore an increased healthspan.
In the Biosphere 2 study in the early 1990s a small number of human subjects unintentionally had limited access to food for about 2 years. Being sealed into a materially closed ecosystem these human subjects were farming and processing their own food, resulting in an energy-limited but nutrient-dense diet. This ER of up to 30 % led to phenotypes similar to those known from ER studies in model organisms(Reference Walford, Harris and Gunion27–Reference Walford, Weber and Panov29). As with rodents and primates, the human subjects displayed decreased body weight and temperature, lowered fasting glucose and insulin levels, and reduced BMR and blood pressure compared with before the study(Reference Walford, Harris and Gunion27, Reference Crowell, Korytko and Morrissey29). A 25 % reduction in energy intake for 6 months in the statistically high-powered CALERIE (Comprehensive Assessment of the Long-term Effects of Reducing Intake of Energy) study by the United States National Institute on Aging (NIA) showed results consistent with these findings. Although there are few data on ER in humans, it is known that the inhabitants of Okinawa Island traditionally consumed a modified Japanese diet containing about 20 % less energy than the Western high-fat diet. Interestingly, the number of centenarians in this population is 4- to 5-fold higher than in Western populations and the Okinawan life expectancy is the highest in the world(Reference Willcox, Willcox and He30). However, in the Okinawan population aged under 65 years, there seems to be a trend towards a lower life expectancy compared with the older generation. This is most probably due to a change from traditional dietary patterns to energy-dense Western-type diets that was initiated in the 1960s(Reference Willcox, Willcox and Todoriki31).
Physical appearance during energy restriction
In addition to a reduction in body weight and fat content(Reference Colman, Anderson and Johnson22, Reference Lane, Mattison and Ingram32), body temperature also decreases under ER(Reference Duffy, Feuers and Leakey33, Reference Mattison, Lane and Roth34). In model organisms on a lifelong ER, a decline in growth and delayed sexual maturity occurred(Reference Mattison, Lane and Roth34) possibly resulting in reduced reproduction rates. In these hypoenergetic-fed animals, a shift from development and reproduction towards maintenance can be observed. Interestingly, changes in activity levels upon ER feeding seem to be species dependent. While rodents undergoing ER showed higher activity levels than ad libitum-fed controls(Reference Weindruch and Walford35), primates displayed mostly unchanged activity patterns compared with the controls(Reference Lane, Mattison and Ingram32, Reference Mattison, Lane and Roth34).
Fig. 1 summarises some of the suggested targets that energy restriction might address.

Fig. 1 Schematic overview of the suggested targets that energy restriction might address.
Blood parameters during energy restriction
As expected from epidemiological studies, in rodent models reduced body weight in the ER groups was accompanied by a decreased risk of developing age-related diseases such as T2DM(Reference Speakman and Mitchell36). In initially overweight as well as in normal-weight subjects, fasting plasma glucose and insulin were reduced(Reference Hwangbo, Gershman and Tu37, Reference Pires, Souza and Vanzela38) whereas adiponectin levels(Reference Zhu, Miura and Lu39) and insulin sensitivity increased(Reference Weindruch, Keenan and Carney40) upon energy restriction. Interestingly, consuming energy-restricted diets even reversed existing diabetic changes like insulin resistance(Reference Lim, Hollingsworth and Aribisala41). ER regimens were also seen to attenuate hypertension(Reference Takatsu, Nakashima and Takahashi42) and hyperlipidaemia(Reference Lezcano, Inigo and Larraga43). The latter observation might also be due to changes in nutrient utilisation. Since, in order to compensate for reduced glucose intake upon ER, gluconeogenesis from lipids and amino acids is induced(Reference Hagopian, Ramsey and Weindruch44). In contrast, as shown in rats, glycolysis is reduced under ER(Reference Feuers, Duffy and Leakey45).
Moreover, improved glucoregulation reduces glycation reactions, in which blood glucose molecules react with amino acids, proteins or nucleic acids in Maillard reactions to form advanced glycation endproducts (AGE)(Reference Hipkiss46). These can bind to their receptors (receptors for AGE; RAGE) in various tissues, thereby inducing renal, vascular or neurological changes that are observed in ageing-related pathologies(Reference Weindruch, Keenan and Carney40, Reference Anisimov, Berstein and Egormin47). Thus, reducing blood glucose levels via ER might contribute to lowering the risk of developing age-dependent diseases such as CVD(Reference Guo, Mitchell-Raymundo and Yang48), neurodegenerative diseases(Reference Yang, Chu and Yin49) and cancer(Reference Weindruch and Walford50, Reference Hursting, Lavigne and Berrigan51).
While insulin secretion is decreased, plasma concentrations of glucocorticoids usually increase when food intake is limited(Reference Sabatino, Masoro and McMahan52). As to be expected from reduced growing rates and the slowing down of maturation, levels of anabolic hormones like leptin and insulin, testosterone, oestradiol(Reference Redman and Ravussin53) and follicle-stimulating hormone(Reference Lane, Mattison and Ingram32) decreased upon ER in several species. However, changes in levels of steroid hormones seem to be dependent on the species, as no changes were seen in primates. In contrast, plasma concentrations of the catabolic adiponectin(Reference Zhu, Miura and Lu39) and of the steroid hormone-binding protein(Reference Cangemi, Friedmann and Holloszy54) tend to be increased by ER. Probably, increased steroid hormone-binding protein levels contribute to the reduced availability of gonadal steroids like testosterone and oestradiol as observed in human subjects undergoing ER regimens(Reference Redman, Martin and Williamson55, Reference Smiarowska, Safranow and Dziedziejko56). Similar to leptin, insulin and testosterone, triiodothyronine (T3) consistently declined in rodents(Reference Sohal and Weindruch57), primates(Reference Roth, Handy and Mattison58) and human subjects(Reference Valenzano, Terzibasi and Genade28, Reference Fontana, Klein and Holloszy59, Reference Heilbronn, de Jonge and Frisard60) upon ER feeding. Remarkably, reduced T3 levels fit well with the reduced body temperatures of ER-fed subjects, as the positive correlation of T3 and body temperature has been known for several years(Reference Wikstrom, Johansson and Salto61). In dwarf mice a deficiency in thyroid-stimulating hormone resulted in hypothyroidism(Reference Brown-Borg, Borg and Meliska62) which causes decreased T3 levels. These mice had increased lifespans that were probably mediated by reduced metabolic rates(Reference Brown-Borg, Borg and Meliska62) and a subsequent reduction in the generation of reactive oxygen species (ROS). However, adaptive thermogenesis might be increased upon ER, as mRNA and protein concentrations of its main regulators uncoupling proteins (UCP) were increased in skeletal muscle of mice(Reference Cadenas, Buckingham and Samec63, Reference Samec, Seydoux and Dulloo64). Because of the increased energy utilisation for mitochondrial heat production – as mediated by increased UCP signalling – ROS production(Reference Duval, Negre-Salvayre and Dogilo65) and the resulting oxidative damage could potentially be decreased.
Cellular processes affected by energy restriction
Energy restriction and oxidative status
Recently, an increasing number of researchers have become interested in the hormesis effect on healthy ageing(Reference Fontana66). This phenomenon describes how a low dose of a stimulus (for example, ROS) induces a positive effect and a high dose of the same stimulus induces a negative effect(Reference Mattson67). As far as ER is concerned, limiting energy supply can be seen as a factor causing mild stress in an organism which activates mechanisms of endogenous stress response, thereby improving the overall protection against stress(68,Reference Ristow and Zarse69). Consistently, enhanced expression of heat shock proteins and antioxidant enzymes under ER support this hypothesis(Reference Sharma and Kaur70). Since accumulated stress is observed in age-related disease, ER might enhance healthspan and lifespan via hormesis(Reference Masoro68).
Although oxidative damage accumulates during ageing(Reference Castello, Froio and Cavallini71) and is characteristic of age-related diseases such as CVD(Reference Lakatta and Levy72), cancer(Reference Valko, Rhodes and Moncol73) and neurodegenerative pathologies(Reference Markesbery74, Reference Browne, Ferrante and Beal75), it is not known whether increased ROS levels are the cause or a consequence of ageing.
ER is assumed to alleviate the age-associated increase in oxidative stress(Reference Sohal and Weindruch57, Reference Gredilla and Barja76), probably via improving endogenous stress response mechanisms(Reference Weindruch, Keenan and Carney40) including several redox-sensitive transcription factors. Indeed, in mice undergoing ER antioxidant enzymes under the transcriptional control of the redox-sensitive transcription factor nuclear factor (erythroid-derived 2) like 2 (Nrf2) such as NQO1 (NAD(P)H dehydrogenase, quinone 1)(Reference Giller, Huebbe and Hennig77) were induced.
Nrf2 protects the organism from the harmful effects of oxidative stress(Reference Zhang and Gordon78). Nrf2 transactivates the expression of genes encoding enzymes that may prevent oxidative damage to cellular structures or remove damaged molecules(Reference Martin-Montalvo, Villalba and Navas79). Apart from NQO1, Nrf2 also induces the transcription of phase II enzymes like glutathione S-transferases in mice(Reference Zhang and Gordon78). Moreover, Nrf2 knock-out mice showed decreased expressions of the antioxidant enzymes catalase, haeme oxygenase 1 (HO1) and superoxide dismutase (SOD) 1(Reference Chan and Kan80). By stimulating proteasomal degradation, Nrf2 can further reduce the burden of oxidatively damaged macromolecules(Reference Wasserman and Fahl81). Interestingly, the expression and transcriptional activity of Nrf2 were shown to decline in tissues of ageing rodents(Reference Suh, Shenvi and Dixon82, Reference Shih and Yen83) and ER was shown to enhance the activity of Nrf2. In mice under ER several Nrf2 target genes were up-regulated, which protected these mice from tumorigenesis. However, this anti-cancer effect was diminished in Nrf2 knock-out mice(Reference Pearson, Lewis and Price84), suggesting a contribution of Nrf2 to the health-promoting effects induced by ER(Reference Sykiotis, Habeos and Samuelson85). Additionally, oxidative damage to macromolecules seems to be reduced upon ER(Reference Gredilla and Barja76), but these beneficial effects often occur only after several months of ER(Reference Faulks, Turner and Else86).
However, in lifespan studies, mutants expressing higher levels of antioxidant enzymes did not always live longer than the controls. While a SOD1 knock-out decreased lifespan in mice(Reference Elchuri, Oberley and Qi87), higher activity of SOD1 (CuZnSOD) did not promote longevity in mice(Reference Huang, Carlson and Gillespie88). Although higher levels of oxidative damaged DNA could be detected in animals with reduced SOD2 (MnSOD) activity, a SOD2 knock-out trial did not reveal different lifespans in transgenic v. control mice(Reference Van Remmen, Ikeno and Hamilton89). Similarly, supplementing mice with antioxidants like glutathione(Reference Lipman, Bronson and Wu90), vitamin C(Reference Selman, McLaren and Meyer91), lipoic acid or coenzyme Q10(Reference Lee, Pugh and Klopp92) did not increase lifespan compared with the untreated controls.
However, in the case of catalase, overexpression of this antioxidant enzyme extended lifespan in mice(Reference Schriner, Linford and Martin93).
Energy restriction and inflammation
While Nrf2, which is thought to be protective against age-related diseases(Reference Sykiotis, Habeos and Samuelson85), was shown to decrease with age(Reference Suh, Shenvi and Dixon82, Reference Shih and Yen83) the transcription factors NF-κB, activator protein-1 and hypoxia inducible factor-1 were shown to be up-regulated age-dependently and in the presence of ROS(Reference Kim, Jung and Yu10). When activated, they contribute to the development of inflammation and associated disorders, for example, arthritis(Reference Chung, Kim and Jung94), cancer, atherosclerosis or neurodegenerative diseases(Reference Baldwin95, Reference Helenius, Hanninen and Lehtinen96).
NF-κB leads to the expression of pro-inflammatory genes encoding cytokines, chemokines and inflammatory cell adhesion which in turn worsen the ROS overload and further promote the activity of NF-κB(Reference Chung, Kim and Kim97). ER consistently decreased the transcriptional activity of NF-κB and hypoxia inducible factor-1(Reference Kang, Kim and Kim98–Reference Horrillo, Gallardo and Lauzurica100). However, the precise mechanism of how ROS increases the activity of these transcription factors is largely unknown(Reference Kim, Jung and Yu10, Reference Li and Karin101). Most likely the reduced load of ROS contributes to a well-balanced cellular redox state, thereby potentially preventing the development of disadvantageous age-associated pathologies(Reference Kim, Jung and Yu10).
Inflammation increases with age(Reference Franceschi and Campisi102) and body weight(Reference Varol, Zvibel and Spektor103) and is often related to tissue injury, organ dysfunction, fibrosis, several chronic diseases and ageing in general(Reference Chung, Cesari and Anton104).
ER increased the anti-inflammatory hormones adiponectin, ghrelin and corticosteroids(Reference Fontana66) and PPAR transcription factors as well as NF-κB inhibitor α(Reference Sung, Park and Yu105, Reference Swindell106) in the plasma of human subjects and rodents. In contrast, NF-κB targets such as inflammation-promoting PG, thromboxanes and other cytokines (TNFα, IL-6, inducible NO synthase, vascular cell adhesion molecule and intercellular adhesion molecule) were decreased(Reference Lane, Mattison and Ingram32, Reference Jung, Lee and Kim99, Reference Higami, Barger and Page107, Reference Kim, Zou and Yoon108). In line with these findings, a diet rich in fat and sugar may increase systemic inflammation(Reference Ford, Giles and Dietz7).
Energy restriction and mitochondrial metabolism
The cellular energy suppliers mitochondria are very sensitive to oxidative damage and are a considerable ROS source themselves(Reference Cadenas and Davies109). Most importantly, these double-membraned organelles contain a subset of different proteins that generate ATP as an energy source for the organism. However, in the oxidation steps of the mitochondrial electron chain, superoxide radicals are formed from leaking electrons and molecular oxygen(Reference Turrens110, Reference Murphy111). Leakage of electrons and therefore basal ROS production increases once the mitochondria become old(Reference Cadenas and Davies109, Reference Harper, Monemdjou and Ramsey112, Reference Hulbert, Pamplona and Buffenstein113) and decreased efficiency in mitochondrial ATP production during ageing leads to less energy and more ROS(Reference Alexeyev, Ledoux and Wilson114), which in turn further damages mitochondria. Similar to other antioxidant defence mechanisms, the activity of the mitochondrial antioxidant defence enzyme SOD2 (MnSOD) also declines with increasing age(Reference Pansarasa, Bertorelli and Vecchiet115), thereby favouring the vicious cycle of less efficient enzymes in the mitochondrial respiratory chain. That leads to an increase in oxidative stress resulting in further mitochondrial destruction.
Thus, it seems useful to promote mitochondrial turnover by enhancing biogenesis of new mitochondria and degradation of old and damaged ones. Biogenesis of mitochondria is a complex multistep interplay of various transcripts and proteins with nuclear, cytosolic and mitochondrial origin(Reference Zhu, Wang and Chu116). It may be activated in response to different stimuli like nutrient supply, endocrine signals, growth factors and changes in temperature(Reference Palikaras and Tavernarakis117). PPAR γ coactivator 1α (PGC1α) is thought to be the key modulator of mitochondrial biogenesis and function, as it regulates the activity of other important factors in mitochondrial biogenesis like nuclear respiratory factors 1 and 2 and oestrogen-related receptors(Reference Dominy and Puigserver118). In turn, nuclear respiratory factors 1 and 2 may induce mitochondrial transcription factor A, which is essential in controlling the expression of mitochondrial genes(Reference Scarpulla119). Oestrogen-related receptors target genes are thought to be involved in nutrient-degrading processes, oxidative phosphorylation and mitochondrial dynamics(Reference Dominy and Puigserver118).
ER was shown to induce mitochondrial biogenesis by enhancing the expression of PGC1α and nuclear respiratory factor 1 as well as their downstream target mitochondrial transcription factor A(Reference Picca, Pesce and Fracasso120). Moreover, PGC1α seems to be involved in mitochondrial function as it enhanced the activity of respiratory chain enzyme complexes III and IV in a cell culture model(Reference Srivastava, Diaz and Iommarini121). In line with these findings, AMP-activated protein kinase (AMPK), which is known to be up-regulated upon ER, was shown to affect mitochondrial development and metabolism positively(Reference Cantó and Auwerx122).
Importantly, ER-induced unselective and selective autophagic degradation may remove old and damaged mitochondria(Reference Massey, Kiffin and Cuervo123), thereby probably reducing oxidative stress. Indeed, ER enhanced mitochondrial turnover in mouse liver, measured as reduced mitochondrial half-life(Reference Miwa, Lawless and von Zglinicki124).
Energy restriction and autophagic processes
Autophagy is an intracellular, lysosomal degradation process that aims at recycling cell organelles, proteins and macromolecules. In all types of autophagy (macroautophagy, microautophagy and chaperone-mediated autophagy) the freight is sequestered and transported to lysosomes, where it is degraded by different hydrolases(Reference Parzych and Klionsky125). While in macro- and microautophagy heterogeneous cytoplasmic content is taken up into vesicles, chaperone-mediated autophagy is able to target, transport and degrade specific proteins(Reference Massey, Kiffin and Cuervo123, Reference Parzych and Klionsky125, Reference Ravikumar, Sarkar and Davies126). However, macroautophagy may also selectively incorporate cargo (such as mitochondria) into autophagosomes, which later fuse with lysosomes to form autolysosomes(Reference Yang and Klionsky127). In the (auto)lysosomes, acidic hydrolases shed organelles and molecules into their components. After transport back to the cytoplasm, these components can be reused for biosynthetic or energy-generating processes(Reference Levine and Klionsky128). Fig. 2 shows the major events of macroautophagy.

Fig. 2 Schematic overview of macroautophagy. A phagophore elongates, wraps around cytosolic components and closes to become an autophagosome. This structure fuses with endosomal vesicles to build late autophagic vesicles and finally fuses with a lysosome, thereby forming an autolysosome in which its inner membrane and its contents are degraded (adapted from Cantó & Auwerx(Reference Cantó and Auwerx122)).
For the best-studied type of autophagy, macroautophagy (in the following referred to as autophagy), it was shown that autophagy-related proteins (ATG) initiate and perform this lysosomal degradation pathway(Reference Parzych and Klionsky125). These ATG are mostly activated under catabolic conditions. Many of them have been shown to be activated by sirtuins (SIRT) and/or inhibited by mammalian target of rapamycin (mTOR) complex 1 (mTORC1), which are induced or repressed by ER, respectively(Reference Powell, Casimiro and Cordon-Cardo129–Reference Alers, Loffler and Wesselborg131). Consistently, ATG(Reference Kim, Jung and Yu10) such as the mammalian ATG8 homologue LC3(Reference Giller, Huebbe and Hennig77) were induced upon ER treatment.
The reason why autophagy is induced upon ER(Reference Morselli, Galluzzi and Kepp8, Reference Giller, Huebbe and Hennig77, Reference Bergamini, Cavallini and Donati132–Reference Rubinsztein, Marino and Kroemer134) is most probably because ER causes a shift from development and reproduction towards maintenance. Under these circumstances autophagy is necessary for recycling of cellular material required for rebuilding essential cell structures and for generating energy(Reference Morselli, Galluzzi and Kepp8, Reference Eisenberg, Knauer and Schauer133, Reference Rubinsztein, Marino and Kroemer134). Interestingly, in lifespan experiments the pro-longevity effect of ER depended on autophagy induction(Reference Bergamini, Cavallini and Donati132). In line with the finding that autophagy induction contributed to the pro-longevity effect of ER, autophagy was shown to decrease during the ageing process(Reference LaRocca, Henson and Thorburn135).
By accelerating the turnover of proteins, membranes and other organelles, autophagy also contributes to maintaining efficiency of peroxisomes, ER and importantly mitochondria(Reference Bergamini, Cavallini and Donati132). Thus, active autophagy could possibly counteract the age-related increase in ROS generation/oxidative stress. This potentially reduces the risk for neurodegenerative diseases like Alzheimer’s, Huntington’s or Parkinson’s in which the accumulation of redundant proteins, followed by functional impairments and the death of post-mitotic neurons are observed. As autophagy removes aggregation-prone redundant or damaged molecules(Reference Donati136) and apoptotic bodies(Reference Rubinsztein, Marino and Kroemer134), increased autophagy might attenuate the progress of neurodegenerative diseases. Indeed, autophagy was found to be dysregulated in patients suffering from Alzheimer’s disease(Reference Salminen, Kaarniranta and Kauppinen137, Reference Wolfe, Lee and Kumar138), Parkinson’s disease(Reference Lynch-Day, Mao and Wang139) and Huntington’s disease(Reference Cortes and La Spada140). Therefore, targeting autophagy induction, for example, by ER, seems to be a valuable strategy for delaying the onset of these diseases and increasing healthspan and lifespan.
Molecular targets of energy restriction
AMP-activated protein kinase
In the case of limited energy supply, ATP levels decline and the AMP:ATP ratio increases which in turn activates, i.e. phosphorylates, the nutrient sensor AMPK(Reference Hardie141). Thus, high levels of phosphorylated AMPK (p-AMPK) are indicative of low energy supply(Reference Zhu, Jiang and McGinley142, Reference Lee, Cao and Mostoslavsky143). Additionally, AMPK appears to be regulated by hormones like leptin and adiponectin, ghrelin and thyroxine(Reference Pimentel, Ropelle and Rocha144).
AMPK phosphorylation leads to activation of various downstream targets such as SIRT1, PGC1α and some forkhead box O (FOXO)(Reference Greer, Oskoui and Banko145–Reference Canto, Jiang and Deshmukh147). This in turn leads to deacetylation of transcription factors and other proteins, biogenesis of mitochondria and stress defence mechanisms. Additionally, AMPK was shown to inhibit the central kinase mTOR, which in its activated complex mTORC1 promotes cell growth and proliferation(Reference Hardie141).
Indeed, AMPK activation has been found to promote longevity in model organisms. Whereas decreased AMPK activity reduced the lifespan of D. melanogaster (Reference Tohyama and Yamaguchi148), an increase in lifespan could be observed in yeast and C. elegans with activated AMPK(Reference Greer, Oskoui and Banko145, Reference Tohyama and Yamaguchi148, Reference Apfeld, O’Connor and McDonagh149).
However, the results concerning the effect of ER on AMPK activity are inconsistent. While some studies found increased p-AMPK in the heart, muscle and liver of lifelong ER-fed mice(Reference Palacios, Carmona and Michan150–Reference Miller, Robinson and Bruss152), others did not observe any changes in p-AMPK levels(Reference Gonzalez, Kumar and Mulligan153).
Interesting insights come from studies that supplemented model organisms with substances that mimic ER. It could be shown that AMPK is necessary for the life-prolonging effect of such ER mimetics(Reference Um, Park and Kang154). Additionally, the deacetylase SIRT1 was also shown to be necessary for ER-mimetic-induced lifespan extension(Reference Price, Gomes and Ling155).
Sirtuins
Various studies have shown that SIRT1 is induced by ER(Reference Han, Zhao and Wang156–Reference Yu, Zhou and Lin158). SIRT1 is part of the sirtuin family – a group of energy-sensing protein deacetylases(Reference Lagouge, Argmann and Gerhart-Hines159). In an energy-dependent manner(Reference Morselli, Maiuri and Markaki160), SIRT1 regulates the activity of many proteins, for example, transcription factors, by changing their state of acetylation(Reference Guarente161). Using NAD+ as a cofactor (the concentration of which rises upon limited nutrient supply), SIRT1 catalyses the transfer of a lysine-bound acetyl group from a protein substrate to NAD+, thereby forming nicotinamide, O-acetyl-ADP-ribose and the deacetylated substrate(Reference Hoff and Wolberger162).
Via deacetylation of histones, SIRT1 might modulate the expression of several genes(Reference Vaquero, Scher and Lee163) involved in energy metabolism. Due to their lysine and arginine residues, histones are charged positively and they form tight complexes with the negatively charged DNA, thereby impeding the RNA polymerase access to the DNA. The acetylation of lysine and arginine residues in the histone weakens its interaction with the DNA(Reference Vaquero, Scher and Lee163, Reference Cameron, Bachman and Myöhänen164), which renders the DNA sequences more susceptible for binding of the polymerase, leading to enhanced gene transcription. Thus, deacetylating histones might down-regulate gene expression(Reference Cameron, Bachman and Myöhänen164).
Additional substrates of SIRT1 include proteins that are involved in mitochondrial biogenesis and turnover. By deacetylating PGC1α and thereby enhancing its activity(Reference Rodgers, Lerin and Haas165), SIRT1 might induce mitochondrial biogenesis(Reference Lopez-Lluch, Irusta and Navas166). Moreover, SIRT1 seems to enhance the activity of ATG5, 7 and 8(Reference Lee, Cao and Mostoslavsky143), which promotes autophagy induction(Reference Lee, Cao and Mostoslavsky143, Reference Morselli, Maiuri and Markaki160, Reference Morselli, Maiuri and Markaki167).
Similar to ER, increased expression of SIRT1 (or its homologues) in yeast, C. elegans, D. melanogaster and mice enhanced lifespan(Reference Kaeberlein, McVey and Guarente168–Reference Satoh, Brace and Rensing171). Mice overexpressing SIRT1 revealed an improved glucose homeostasis, including increased insulin sensitivity and were protected from developing T2DM(Reference Liang, Kume and Koya172). High-fat diet-fed mice with increased SIRT1 expression seemed to be resistant to the metabolic syndrome(Reference Pfluger, Herranz and Velasco-Miguel173). Moreover, SIRT1 was shown to prevent metabolic syndrome-induced tumour development(Reference Herranz, Muñoz-Martin and Cañamero174). Via interacting with and inhibiting the binding sites of PPARγ (a transcription factor that regulates lipid metabolism), SIRT1 reduces the expression of its target genes in white adipocytes(Reference Tamori, Masugi and Nishino175, Reference Picard, Kurtev and Chung176). Thus, SIRT1 may also inhibit lipid storage and enhance fat mobilisation in white adipose tissue upon ER(Reference Picard, Kurtev and Chung176).
Conversely, some studies found that ER increased lifespan even in the absence of SIRT1 and other SIRT(Reference Jiang, Wawryn and Shantha Kumara177, Reference Smith, McClure and Matecic178). It could be possible that the signalling proteins of the interdependent network SIRT1, AMPK and PGC1α can in part compensate for each other(Reference Canto, Jiang and Deshmukh147).
PPARγ coactivator 1α
The key target of Sirt1 and AMPK, the co-activator of the transcription factor PPARγ PGC1α(Reference Jager, Handschin and St-Pierre146, Reference Kanfi, Peshti and Gozlan179), is also central for mediating the ER-induced lifespan-enhancing effect(Reference Lopez-Lluch, Hunt and Jones180). When activated, PGC1α might even prevent age-related undesirable changes(Reference Hepple, Baker and McConkey181, Reference Anderson and Prolla182) in skeletal and heart muscle and in adipose tissue. Apart from its role in mitochondrial biogenesis and function(Reference Lopez-Lluch, Irusta and Navas166) in all cell types, in skeletal muscle PGC1α is thought to affect the type of muscle fibre(Reference Lin, Wu and Tarr183). Furthermore, PGC1α stimulates adaptive thermogenesis in brown adipose tissue(Reference Puigserver, Wu and Park184). PGC1α activity appears to prevent loss of mitochondrial function in muscles, protects from distributional and functional changes of adipose tissue and affects the energy metabolism by favouring lipids instead of carbohydrates as a main energy source(Reference Anderson and Prolla182).
While some ER studies found that nuclear PGC1α levels (and subsequent increase in mitochondrial biogenesis) increased under limited nutrient supply(Reference Hepple, Baker and McConkey181, Reference Lee, Allison and Brand185), others did not observe any differences in nuclear PGC1α protein concentrations in rodent tissues such as heart, liver, white adipose tissue and brain(Reference Hancock, Han and Higashida186). However, in mice the SIRT1-mediated activation of PGC1α also led to increased gluconeogenesis, enhanced glucose mobilisation and reduced levels of glycolysis(Reference Rodgers, Lerin and Haas165).
Forkhead box O
FOXO transcription factors were also shown to be activated by SIRT1(Reference Rodgers, Lerin and Haas165). FOXO are involved in stress defence and energy metabolism(Reference Calnan and Brunet187, Reference Finkel, Deng and Mostoslavsky188) and induce the transcription of genes that promote autophagy and cell death(Reference Brunet, Sweeney and Sturgill189, Reference Zhao, Yang and Liao190).
Apart from deacetylation, which activates FOXO, they can be regulated negatively by phosphorylation(Reference Plas and Thompson191). After being phosphorylated, FOXO are shuttled from the nucleus into the cytoplasm, which reduces the expression of FOXO target genes(Reference Tazearslan, Cho and Suh192).
Insulin-like growth factor 1
The IGF-1 signalling pathway is also negatively affected under ER(Reference Lane, Mattison and Ingram32, Reference Bluher, Kahn and Kahn193). The reduced IGF-1 signalling observed under ER(Reference Berryman, Christiansen and Johannsson194) is usually accompanied by lowered growth hormone (GH) concentrations(Reference Lane, Mattison and Ingram32), since GH promotes IGF expression(Reference Brown-Borg, Borg and Meliska62). An ER-induced reduction in plasma levels of free IGF-1 was shown to be pro-apoptotic and anti-proliferative(Reference Dunn, Kari and French195) and is therefore potentially chemopreventive.
Indeed, GH(Reference Steger, Bartke and Cecim196) and IGF-1 receptor(Reference Suh, Atzmon and Cho197, Reference Holzenberger, Dupont and Ducos198) deficiencies were shown to increase lifespan, whereas GH overexpression led to premature ageing and shortened lifespan in mice(Reference Steger, Bartke and Cecim196). Dwarf mice that display reduced IGF-1/GH signalling lived considerably longer compared with normal-sized wild-type mice, mainly because they had a reduced incidence of neoplasms(Reference Ikeno, Bronson and Hubbard199). Furthermore, it was shown that low IGF-1 levels were related to survival in long-lived humans(Reference Milman, Atzmon and Huffman200).
Additionally to decreased levels of growth factors and hormones, the reduction of insulin secretion upon ER feeding could also account for its chemopreventive effect. Hyperinsulinaemia is strongly related to high levels of oxidative stress(Reference Facchini, Hua and Reaven201) and reduced rates of autophagy(Reference Liu, Han and Cao202), leading to increased generation of damaged macromolecules and decreased degradation of damaged proteins.
Another consequence of increased IGF-1 signalling is the induction of phosphoinositide-dependent protein kinase, which in turn activates protein kinase B by phosphorylation(Reference Fleming-Waddell, Olbricht and Taxis203), thereby activating mTOR.
Mammalian target of rapamycin
mTOR is a seronine/threonine protein kinase that is involved in several regulatory processes. It is suggested that it influences cell growth, including proliferation, transcription and protein synthesis, as well as cell survival(Reference Hay and Sonenberg204).
Due to its central role in proliferation and cellular growth processes, mTOR has long been studied as a molecular target for cancer therapy(Reference Ciuffreda, Di Sanza and Incani205). Inhibiting mTOR activity genetically or pharmacologically increased lifespan in yeast(Reference Powers, Kaeberlein and Caldwell206, Reference Kaeberlein, Powers and Steffen207), C. elegans (Reference Jia, Chen and Riddle208, Reference Vellai, Takacs-Vellai and Zhang209) and D. melanogaster (Reference Kapahi, Zid and Harper210).
Consistent with decreased IGF-1 and GH signalling(Reference Ikeno, Bronson and Hubbard199), which are upstream regulators of mTOR, mTOR signalling is also reduced in dwarf(Reference Sharp and Bartke211) and ER-fed mice(Reference Sun, Sadighi Akha and Miller212). Inhibition of mTOR leads to autophagy induction(Reference Ravikumar, Vacher and Berger213, Reference Toth, Sigmond and Borsos214) possibly via dephosphorylation of ATG13 and the mammalian Atg1 homologues Unc-51 like autophagy activating kinases 1 and 2, which are essential for mTOR-dependent autophagy(Reference Fleming, Noda and Yoshimori130, Reference Alers, Loffler and Wesselborg131).
Limitations and adverse effects of energy restriction
Evaluating ER as a potential pro-longevity treatment, it is necessary to keep in mind some of its adverse effects (see Table 1). First of all, it seems almost infeasible to follow a lifelong ER regimen that requires a reduction of energy intake of up to 40 %(Reference Duffy, Seng and Lewis215) without being malnourished at any time. Another critical issue in ER experiments might be decreased bone mineral density as observed in primates and rodents(Reference Lane, Mattison and Ingram32) as well as in a long-term ER study in human subjects(Reference Villareal, Kotyk and Armamento-Villareal216). Deficiencies in skin wound healing – seen in restrictively fed rats(Reference Hunt, Li and Zhu217) – and decline in concentrations of gonadal steroids(Reference Cangemi, Friedmann and Holloszy54) could impair health-related quality of life. Furthermore, symptoms such as starving, cold and reduced libido persist during long-term ER and the social aspect of eating food should not be neglected(Reference Speakman and Mitchell36). Importantly, ER-fed old mice seem to be more susceptible to infections(Reference Goldberg, Romero-Aleshire and Renkema218), which could even decrease lifespan.
Table 1 ‘Side effects’ of energy restriction

An alternative to ER is the ‘alternate day fasting’ regimen. In this modified ER concept, dietary intake is partially or completely limited every other day, whereas food consumption remains unrestricted on the remaining days(Reference Goodrick, Ingram and Reynolds219). Although it could be expected that a higher food intake in ad libitum phases compensates or even overcompensates for energy shortage during the restriction days, several health-promoting effects were seen in alternate day fasting compared with control groups with unlimited food access(Reference Johnson, Laub and John220–Reference Mager, Wan and Brown223). However, this nutritional concept also seems hard to follow in modern society and possible side effects need to be studied in more detail.
Energy restriction mimetics
Because of its limitations, alternatives to ER that could prevent or retard the onset of ageing-related pathologies to a similar extent as ER are being sought. Since it takes very long to generate data on the influence of dietary regimens, supplements or pharmacological substances on human lifespan and age-related diseases there is only limited information on general ageing patterns in specific organs and tissues. Using broad-range microarrays, gene expression profiles associated with the ageing process have been identified. By comparing these patterns of aged individuals to subjects undergoing ER and those supplemented with potential ERM, the anti-ageing potential of candidate substances can easily be assessed(Reference Lee, Pugh and Klopp92). Results of ongoing studies identifying potential ERM are published regularly by the NIA Interventions Testing Program. A comprehensive review on ER effects with a focus on epigenetic changes was recently published by Chung et al. (Reference Chung, Kim and Park224). Moreover, Ingram et al. (Reference Ingram, Zhu and Mamczarz225) have established criteria that should be met before a substance is considered an ERM. First, candidate substances should not significantly influence long-term dietary intake in order to prevent positive results merely because of reducing energy consumption. Second, the candidate substance should induce metabolic, hormonal and physiological changes that are comparable with the effects of ER. And third, it should induce an ER-like stress response. It is hypothesised that through meeting these three criteria, dietary supplementation with the candidate substance may have a positive influence on healthspan and lifespanReference Smith, Nagy and Allison(226). According to Selman, the elongation of healthspan by putative ERM candidates should be more emphasised(Reference Selman227).
The criterion proposed by Lane et al. (Reference Lane, Roth and Ingram228) resembles these points. They state that without altering food intake the potential ERM should target energy metabolism since this seems to be the underlying mechanism of the beneficial ER effects.
Putative energy restriction mimetic candidate substances
ERM target various molecules and processes that have been described in the ‘Cellular processes affected by energy restriction’ section. A putative ERM that targets glucoregulation and inhibits glycolysis is the glucose analogue 2-deoxy-d-glucose (2DG)(Reference Speakman and Mitchell36). Recently, glucosamine has been discussed as mimicking a low-carbohydrate diet and thus potentially acting as an ERM(Reference Weimer, Priebs and Kuhlow229).
Further ERM candidates are the biguanides that have been used in the treatment of T2DM since they lower insulin/IGF-1 signalling and activate AMPK(Reference Speakman and Mitchell36). While formerly phenformin and butformin were used alongside metformin, nowadays only metformin is approved for T2DM treatment. Thus, despite studies showing a prolonged lifespan in phenformin- and butformin-supplemented rats(Reference Ingram, Anson and de Cabo230, Reference Smith, Elam and Mattison231), metformin currently is the best studied biguanide in terms of mimicking ER. Oxaloacetate seems to activate AMPK-related pathways, thereby increasing lifespan in a nematode model. However, supplementation trials in mice did not reveal any pro-longevity effect of oxaloacetate(Reference Ingram and Roth232).
Another substance class suggested as possessing pro-longevity effects are anti-lipolytic compounds such as the nicotinic acid derivative acipimox(Reference Donati, Cavallini and Carresi233) or 3,5- dimethylpyrazole(Reference Straniero, Cavallini and Donati234). Additionally to their lowering effect on plasma lipids, they are thought to be glucose- and insulin-decreasing as well as autophagy-inducing agents. These observations all point to potential ER-like effects. Unfortunately, studies looking at lifespan upon/after treatment with anti-lipolytic drugs are rare(Reference Speakman and Mitchell36). Further ERM candidates can be found among SIRT stimulators, particularly stilbenes. Systemic SIRT1 activators are known for their metabolism-stimulating and body fat-reducing effects(Reference Alcaín and Villalba235). The best-studied member of this group is resveratrol (RSV), which has been shown to increase lifespan in some species. Nicotinamide might also be considered a putative ERM since it was shown to activate SIRT. Interestingly, nicotinamide and RSV induce autophagy which might contribute to their ER-mimicking potential(Reference Marino, Pietrocola and Madeo236). Autophagy-inducing agents might also promote ER-like phenotypes independently of SIRT regulation. Spermidine (SPD) was shown to induce autophagy and thereby increase lifespan in some species without affecting SIRT(Reference Eisenberg, Knauer and Schauer133). Another target molecule for ERM is the proliferation and cell growth-promoting kinase mTOR. Indeed, supplementation experiments with the pharmacological mTOR inhibitor rapamycin produced promising results.
Furthermore, agents that affect mitochondrial biogenesis or the circadian rhythm may mediate ER-mimicking effects. Antioxidants such as lipoic acid that reduce ROS production by improving mitochondrial performance or increasing the production of endogenous antioxidants are also interesting putative ERM. Because of its influence on the circadian rhythm, melatonin might be helpful in managing body weight and preventing chronic diseases and premature deaths(Reference Navarro-Alarcón, Ruiz-Ojeda and Blanca-Herrera237).
Sometimes anorectics like amphetamines are discussed as ERM candidates(Reference Speakman and Mitchell36). But due to their considerable reduction of food and consequently energy intake, anorectics cannot be considered ERM according to the criteria mentioned above(Reference Smith, Nagy and Allison226, Reference Lane, Roth and Ingram228, Reference Ingram, Anson and de Cabo230).
Postulated mechanisms and potential limitations of the best-studied and most promising energy restriction mimetics
The following section will focus on postulated mechanisms and potential limitations of the best-studied and most promising ERM as found in recent literature (for a summary, see Table 2).
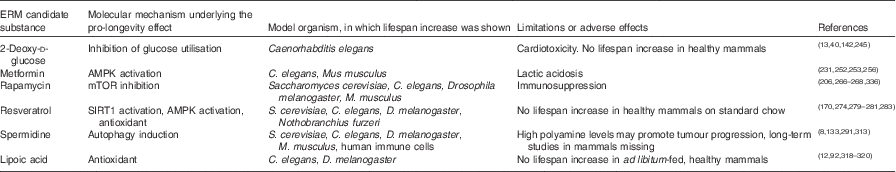
Table 2 Potential energy restriction mimetic (ERM) candidate substances, their underlying molecular mechanisms, organisms in which the substances prolonged lifespan and limitations or adverse effects

AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin, SIRT1, sirtuin 1.
2-Deoxy-d-glucose
2DG (Fig. 3) was one of the first substances discussed as a possible ERM(Reference Lane, Ingram and Roth238). As a glucose analogue it differs from glucose only in that it lacks one hydroxyl group at C2Reference Minor, Smith and Sossong(13). After intestinal absorption and entering the circulation, 2DG is taken up into cells using the same transporters as glucose. 2DG is phosphorylated immediately by a hexokinase and accumulates intracellularly since it cannot be metabolised by phospho-hexose isomerase(Reference Weindruch, Keenan and Carney40). Thereby, it competitively inhibits glucose utilisation, reduces the amounts of available energy(Reference Brown239) and might consequently mimic the effects of ER(Reference Minor, Smith and Sossong13).

Fig. 3 Chemical structure of 2-deoxy-d-glucose.
Indeed, in rodents, adding 2DG to the diet led to ER-similar phenotypes(Reference Lane, Mattison and Ingram32) including decreased body weight, blood glucose(Reference Yao, Chen and Mao240), insulin(Reference Wan, Camandola and Mattson241), body temperature(Reference Ingram and Roth242) and heart rate(Reference Wan, Camandola and Mattson241). Moreover, 2DG was shown to induce protection against oxidative stress(Reference Lee, Bruce-Keller and Kruman243).
Additionally, application of 2DG elevated protein levels of SIRT1 and p-AMPK in MCF-7 cancer cells(Reference Zhu, Jiang and McGinley142). Since the inhibition of glycolytic processes by 2DG is followed by a limitation of cellular energy supply, ATP levels are reduced and subsequently the AMP:ATP ratio is increased leading to AMPK activation which increases the NAD+:NADH ratio and activates SIRT1(Reference Canto, Gerhart-Hines and Feige244).
In fact, Schulz et al. (Reference Schulz, Zarse and Voigt245) reported enhanced mean and maximum lifespan of C. elegans upon 2DG supplementation. However, these results could not be verified in mammals. Long-term studies in rodents revealed cardiotoxic effects of 2DG treatment at concentrations which were necessary for ER-like effects(Reference Minor, Smith and Sossong13). In a long-term feeding trial in rats, 2DG was shown to reduce lifespan dose-dependentlyReference Minor, Smith and Sossong(13). However, at lower concentrations which did not seem toxic, 2DG exerted health-promoting effects in a 5-week supplementation study in tumour-prone rats where it inhibited cancer growth(Reference Zhu, Jiang and McGinley142).
In conclusion, 2DG-induced ER-like phenotypes in laboratory animals are possibly outweighed by its cardiomyopathic effects observed in some studies. Toxicity thresholds should therefore be considered when planning long-term or even lifelong 2DG supplementation studies. Thus, further studies on dose-dependent efficiency and toxicity are needed.
Metformin
The biguanide metformin (Fig. 4) has been used for treating diabetes for a long time(Reference Correia, Carvalho and Santos246). Due to its potential of inducing ER-like effects, metformin has been considered an ERM candidate substance(Reference Smith, Elam and Mattison231). Metformin was shown to suppress gluconeogenesis and promote insulin sensitivity and glycolysis in diabetic subjects(Reference Correia, Carvalho and Santos246, Reference Radziuk, Bailey and Wiernsperger247). Moreover, intestinal glucose absorption and plasma glucose and lipid concentrations were decreased(Reference Testa, Biasi and Poli248).

Fig. 4 Chemical structure of metformin.
In different tumour-prone rodent models and in diabetic patients incidence and progression of cancer were decreased by metformin(Reference Speakman and Mitchell36, Reference Smith, Elam and Mattison231, Reference Libby, Donnelly and Donnan249, Reference Berstein250). Additionally, risk factors for the development of CVD were reduced upon metformin treatment(Reference Nagi and Yudkin251). Most probably, the delay in the onset of chronic diseases such as cancer and hypertension is the main reason for the longevity-promoting effect seen in C. elegans and mice(Reference Smith, Elam and Mattison231, Reference Anisimov, Berstein and Egormin252).
Interestingly, metformin caused a reduction in body weight in several studies without significantly decreasing energy intake(Reference Martin-Montalvo, Mercken and Mitchell6, Reference Smith, Elam and Mattison231). Since a reduction in body weight is associated with an increased AMPK activation, it seems reasonable to assume a contribution of AMPK to the beneficial effects of metformin. Indeed, the levels of p-AMPK were found to be elevated upon metformin supplementation(Reference Martin-Montalvo, Mercken and Mitchell6, Reference Ben Sahra, Le Marchand-Brustel and Tanti253). Potential longevity-promoting effects of AMPK and its downstream targets have been described earlier in this article.
Metformin probably addresses further targets that also contribute to the observed increase in lifespan(Reference Testa, Biasi and Poli248). In rodent models metformin was seen to mimic the hepatic and muscular transcriptional ER response(Reference Martin-Montalvo, Mercken and Mitchell6, Reference Dhahbi, Mote and Fahy254, Reference Onken and Driscoll255). These gene expression data indicate improved mitochondrial function, glucose and lipid metabolism, reduced apoptotic rates and inflammation, as well as enhanced stress response. In particular, mRNA expression and nuclear protein levels of the central antioxidant transcription factor Nrf2 were found to be up-regulated. Additionally, mRNA concentrations of its downstream targets SOD, NQO1 and NQO2 were elevated compared with the control animals. In contrast, expression of pro-inflammatory genes like NF-κB seemed decreased after metformin treatment(Reference Martin-Montalvo, Mercken and Mitchell6).
Despite these promising effects regarding the delay of the ageing process, some impairments of chronic metformin application have to be taken into account. Metformin is suggested as causing lactic acidosis when applied at higher doses for a longer period(Reference Holst, Eldrup and Guldstad256). Indeed, microarray analyses revealed increased mRNA concentrations of cytosolic lactate dehydrogenase (a key enzyme in lactic acid generation) in the liver and muscle of metformin-supplemented mice(Reference Martin-Montalvo, Mercken and Mitchell6). However, metformin was applied at doses that were higher than the doses used in human subjects and the toxicity of metformin seems to depend on the dose. Current meta-analyses and reviews on metformin-treated T2DM patients concluded that the increased incidence of lactic acidosis more probably resulted from an underlying systemic dysfunction than from the metformin treatment(Reference Salpeter, Greyber and Pasternak257, Reference Kruse258). Moreover, metformin supplementation may be contraindicated in individuals suffering from renal diseases(Reference Nisbet, Sturtevant and Prins259) since metformin induced renal failure in rodents when applied at high concentrations. Despite metformin being a promising ERM candidate, supplementation studies in healthy mammals have not always observed a clear lifespan-enhancing effect(Reference Smith, Nagy and Allison226). For example, a study in healthy rats supplemented with metformin failed to show a pro-longevity effect(Reference Smith, Elam and Mattison231), whereas a study in C57BL/6 mice showed longevity-promoting effects after metformin treatment(Reference Martin-Montalvo, Mercken and Mitchell6).
Rapamycin
Rapamycin (Fig. 5), a complex macrolide antibiotic, has been used because of its immunosuppressive effects(Reference Wullschleger, Loewith and Hall260) after organ transplantations and because of its antiproliferative properties in cancer treatment(Reference Garber261, Reference Crespo and Hall262). It has long been known that rapamycin exerts its antiproliferative effects via inhibition of mTOR signalling(Reference Heitman, Movva and Hall263). Possibly, reduced mTOR activation decreases cancer risk and therefore prolongs survival in rapamycin-treated subjects. After discovering that deleterious TOR mutations in yeast promoted longevity, rapamycin was suggested as being an ERM in 2006(Reference Powers, Kaeberlein and Caldwell206). Indeed, it has been shown to decelerate senescence in vitro (in non-cancerous cell lines) and to possess lifespan-enhancing properties in several model organisms such as Saccharomyces cerevisiae (Reference Powers, Kaeberlein and Caldwell206, Reference Alvers, Wood and Hu264), C. elegans, D. melanogaster (Reference Fontana, Partridge and Longo265, Reference Cuervo266) and mice(Reference Cuervo266–Reference Miller, Harrison and Astle268).

Fig. 5 Chemical structure of rapamycin.
How rapamycin interferes with mTOR is still under discussion. It has been hypothesised that rapamycin could bind to the heterodimer of the FK506 binding protein (FKBP)-type peptidyl-prolyl cis-trans-isomerase and the FKBP–rapamycin-associated protein. As such a heterotrimer, rapamycin might form complexes with mTOR, thereby inhibiting the formation of the complex mTORC1 and consequently cell proliferation and cell growth(Reference Stanfel, Shamieh and Kaeberlein269). Of interest, via inhibition of mTORC1, rapamycin was also shown to activate autophagy by up-regulating ATG1(Reference Cuervo266, Reference Mizushima270, Reference Calabrese, Cornelius and Dinkova-Kostova271). Similar to findings from ER studies, prolonged lifespan induced by low doses of rapamycin was shown to depend on autophagy since rapamycin failed to enhance survival in atg1- and atg7-deficient yeast(Reference Alvers, Wood and Hu264). Several beneficial outcomes of rapamycin administration, which may contribute to its pro-longevity effect, were observed in clinical and pre-clinical studies; namely, rapamycin seemed to alleviate the ageing-related diseases T2DM, the metabolic syndrome, atherosclerosis, neurodegeneration and some kinds of cancer(Reference Bove, Martinez-Vicente and Vila272, Reference Blagosklonny273). However, body weight does not appear to be affected by rapamycin administration in mice fed standard diets(Reference Harrison, Strong and Sharp267).
A limitation for rapamycin use as an ERM in humans results from its immunosuppressive action. While mice housed in specific pathogen-free cages are unlikely to acquire infections despite being supplemented with immunosuppressants, humans may not benefit from rapamycin due to severe bacterial or viral diseases(Reference Smith, Nagy and Allison226). Moreover, animals treated with high rapamycin doses suffered from disturbed lipid or glucose homeostasis, skin irritations, anaemia or impaired wound healing(Reference Testa, Biasi and Poli248). Currently, studies are being carried out by the NIA to evaluate the most effective dose for promoting longevity in rodents(Reference Miller, Harrison and Astle268).
Resveratrol
RSV (Fig. 6), a naturally occurring stilbene with antioxidant capacity(Reference Afaq and Mukhtar274), is mainly found in the skin of red grapes and therefore in red wine(Reference Agarwal and Baur275, Reference Vuong, Franco and Zhang276). Considerable amounts can also be detected in the roots of the medical plant Japanese knotweed(Reference Baur and Sinclair277) and lower amounts in other fruits(Reference Zou, Carey and Liedo278). In a NIA in vitro screening programme, RSV was identified as a SIRT1 activator in 2003. As SIRT1 seems to be a crucial player in ER-mediated lifespan extension, RSV was additionally tested for its lifespan-enhancing effects in S. cerevisiae. Due to the promising results of this trial(Reference Howitz, Bitterman and Cohen279) several studies have been conducted to verify the lifespan-enhancing properties of RSV. Indeed, RSV has shown survival enhancing effects in yeast, C. elegans, some D. melanogaster experiments(Reference Wood, Rogina and Lavu170), Nothobranchius furzeri (Reference Valenzano, Terzibasi and Genade280) and high-fat diet-fed mice(Reference Baur, Pearson and Price281). Additionally, Morselli et al. proposed that RSV-mediated SIRT1 activation was followed by the induction of autophagy, which appears to be important for the lifespan-enhancing effect of ER(Reference Morselli, Galluzzi and Kepp8).

Fig. 6 Chemical structure of resveratrol.
However, in other fly studies(Reference Zou, Carey and Liedo278, Reference Bass, Weinkove and Houthoofd282) and in mice fed a standard chow RSV did not prolong lifespan(Reference Miller, Harrison and Astle268, Reference Pearson, Baur and Lewis283, Reference Strong, Miller and Astle284). Nevertheless, in standard chow-fed mice supplemented with RSV several tissues revealed ER-like gene expression patterns, and anti-inflammatory, cardio- and osteoprotective effects as well as improved locomotor activity were observed(Reference Pearson, Baur and Lewis283).
While it seems that SIRT1 might be responsible for the beneficial effects of RSV(Reference Baur, Pearson and Price281) the exact mechanism of SIRT1 remains unclear. RSV may be binding to the regulatory(Reference Pan, Yuan and Brent285) N-terminal subunit of SIRT1. Subsequently, a conformational change could take place, which in turn could lead to an enhanced SIRT1 deacetylation activity(Reference Howitz, Bitterman and Cohen279). Thus, the red wine polyphenol would seem to be an allosteric SIRT1 activator(Reference Morselli, Galluzzi and Kepp8, Reference Howitz, Bitterman and Cohen279).
Intriguingly, there are reports that the observed increase in SIRT1 deacetylase activity due to RSV could have been a result of a methodological error(Reference Pacholec, Bleasdale and Chrunyk286, Reference Kaeberlein, McDonagh and Heltweg287). If the enzyme activity measurement is not performed with fluorochrome-conjugated substrates (as it usually is) but with natural substrates, RSV does not increase SIRT1 activity(Reference Pacholec, Bleasdale and Chrunyk286).
However, Park et al. showed that RSV inhibits cAMP-degrading phosphodiesterases, which in turn leads to an activation of the AMPK pathway. They further argue that this increases NAD+ levels and that SIRT1 activation is a consequence of a RSV-mediated rise in NAD+ levels(Reference Park, Ahmad and Philp288). These findings could explain why Pacholec et al. could not detect SIRT1 activation when measuring the enzyme activity in vitro (Reference Pacholec, Bleasdale and Chrunyk286).
Interestingly, experiments in knockout animals have shown that apart from SIRT1(Reference Wood, Rogina and Lavu170) AMPK is also necessary for the pro-longevity effect of RSV(Reference Um, Park and Kang154). The beneficial effects of RSV on insulin sensitivity, motor function, mitochondrial performance(Reference Lagouge, Argmann and Gerhart-Hines159, Reference Baur, Pearson and Price281) as well as decreased body weight(Reference Um, Park and Kang154, Reference Lagouge, Argmann and Gerhart-Hines159) were not seen in AMPK-deficient animals(Reference Um, Park and Kang154). A recent publication has described a mechanism for AMPK activation by RSV in vitro that does not depend on SIRT1 but on the tyrosyl transfer-RNA synthetase. RSV may fit into the active site of the tyrosyl transfer-RNA synthetase, thereby leading to nuclear translocation of this enzyme and consequently activation of the poly(ADP-ribose) polymerase 1 which in turn could activate downstream targets such as AMPK, FOXO and SIRT. Intriguingly, this happened at up to 100-fold lower doses than in previous studies(Reference Sajish and Schimmel289).
In contrast to many other ERM candidate substances, hardly any adverse effects have been reported for RSV so far. Only a study applying very high RSV doses observed cases of premature death(Reference Crowell, Korytko and Morrissey290). However, it needs to be kept in mind that the efficiency of RSV in reducing mortality rates in healthy mammals and the appropriate dose of RSV for supplementation are still under discussion.
Spermidine
Spermidine (SPD; Fig. 7) is a naturally occurring polyamine that is essential for various cellular processes. Along with other endogenous polyamines such as spermine and putrescin it was shown to modulate various cellular processes such as proliferation, differentiation and cell death(Reference Soda, Dobashi and Kano291).

Fig. 7 Chemical structure of spermidine.
Looking at particular polyamines, putrescine and cadaverine turn out to be rather negatively annotated molecules since they are enhanced in tissues in chronic diseases such as cancer(Reference Scalabrino and Ferioli292), Parkinson’s(Reference Paik, Ahn and Lee293) and pancreatitis(Reference Jin, Raty and Minkkinen294). In contrast, SPD seems to be protective against age-related pathological changes(Reference Eisenberg, Knauer and Schauer133). Spermine and SPD have already been described as exerting anti-inflammatory effects(Reference ter Steege, Forget and Buurman295, Reference Soda, Kano and Nakamura296). Additionally, their levels were shown to be reduced in Alzheimer’s disease(Reference Seidl, Beninati and Cairns297). Thus, the restoration of SPD and spermine levels might ameliorate Alzheimer’s pathology. Moreover, due to its anti-inflammatory properties, SPD has been suggested for the treatment of multiple sclerosis(Reference Guo, Harada and Namekata298) and sepsis(Reference Zhu, Ashok and Li299).
As the endogenous synthesis of SPD declines with age(Reference Morgan300, Reference Minois, Carmona-Gutierrez and Madeo301), supplementation with SPD could increase the plasma concentrations at a higher age(Reference Soda, Dobashi and Kano291). Food rich in SPD includes soya and other beans, green tea and mushrooms(Reference Bardócz, Grant and Brown302, Reference Amendola, Cervelli and Fratini303). SPD rich diets are consumed traditionally in Asian(Reference Binh, Soda and Maruyama304) and Mediterranean(Reference Binh, Soda and Kawakami305) regions.
Recently, elevated SPD relative to total polyamine concentrations have been observed in the plasma of centenarians compared with younger counterparts(Reference Pucciarelli, Moreschini and Micozzi306). Consistently, SPD supplementation in aged mice(Reference Soda, Dobashi and Kano291), S. cerevisiae, C. elegans, D. melanogaster and human immune cells(Reference Eisenberg, Knauer and Schauer133) increased lifespan.
Some researchers assume SPD to exert its longevity-promoting effects via induction of autophagy(Reference Morselli, Galluzzi and Kepp8, Reference Eisenberg, Knauer and Schauer133). Autophagy is known to be reduced at a higher age(Reference Rubinsztein, Marino and Kroemer134, Reference Jia and Levine307), and seems to counteract senescence when induced(Reference Jia and Levine307, Reference Melendez, Talloczy and Seaman308).
In vitro experiments revealed that SPD treatment inhibited the activity of histone acetyltransferases in yeast and mouse liver extracts(Reference Morselli, Galluzzi and Kepp8, Reference Eisenberg, Knauer and Schauer133). Gene expression depends on the acetylation state of histones. The negatively charged chromatin can be wrapped around the histones more tightly when more of the lysine and arginine residues in the histone molecules remain deacetylated, i.e. positively charged. Thus, histone acetylation and deacetylation may alter the accessibility of certain DNA sections to transcriptionally active enzymes(Reference Jenuwein and Allis309). Therefore, a high number of deacetylated histones – as observed after SPD treatment(Reference Eisenberg, Knauer and Schauer133) – leads to modified expression of several genes(Reference Jenuwein and Allis309). Recently it has been reported that SPD-treated yeast expressed Atg7, 11 and 15, which are involved in autophagy induction, at enhanced levels(Reference Morselli, Galluzzi and Kepp8). Indeed, SPD failed to increase lifespan in atg-deficient yeast(Reference Eisenberg, Knauer and Schauer133).
However, in D. melanogaster, recent data suggest a contribution of non-autophagic mechanisms to the improved response to oxidative stress upon SPD supplementation although these additional underlying signalling pathways have not been fully elucidated(Reference Minois, Carmona-Gutierrez and Bauer310, Reference LaRocca, Gioscia-Ryan and Hearon311).
SPD is still a very young topic in longevity research; therefore studies on potential negative side effects are rare. However, elevated concentrations of polyamines are thought to be related to an increased incidence of cancer. Possibly, oxidative damage, caused by oxidation of polyamines in general, contributes to this effect(Reference Amendola, Cervelli and Fratini303).
Potential limitations in the use of SPD as an ERM result from observations that increased polyamine concentrations caused enhanced tumour progression(Reference Sarhan, Knodgen and Seiler312–Reference Clifford, Morgan and Yuspa314). In fact, putative anti-cancer agents inhibit polyamine metabolism(Reference Amendola, Cervelli and Fratini303). However, in these studies polyamines were applied to model organisms with existing tumours or cells that had undergone oncogenic transformation(Reference Soda, Dobashi and Kano291). Up to now, there is no direct evidence for cancer-promoting effects of enhanced SPD intake in healthy animals. So far, however, there are no long-term studies in healthy mammals.
Lipoic acid
The disulfide derivative of octanoic acid is well known for its antioxidant activity that is also exerted by its reduced form dihydrolipoic acid(Reference Packer, Witt and Tritschler315). Apart from its antioxidant effect, lipoic acid (Fig. 8) is an essential cofactor for certain enzyme complexes, for example, pyruvate dehydrogenase and α-ketoglutarate dehydrogenase(Reference Packer, Tritschler and Wessel316).

Fig. 8 Chemical structure of R-α-lipoic acid.
Due to the beneficial effects of antioxidants in general, it seems worth examining the healthspan- and lifespan-extending properties of lipoic acid.
Lipoic acid exists as two stereoisomers, R-α-lipoic acid and S-α-lipoic acid. The biologically more active stereoisomer R-α-lipoic acid can be synthesised endogenously from fatty acids (octanoic acid) and a sulfur source (cysteine) or taken up with food(Reference Packer, Kraemer and Rimbach317). Exogenously supplied lipoic acid is mostly bound to lysine. As lipoyllysin it can be found in vegetables, for example, spinach, broccoli and tomatoes, as well as in animal tissues like liver, heart and kidney(Reference Merry, Kirk and Goyns12).
According to some popular ageing theories, free radicals are probably a major reason for the development of ageing-associated diseases(Reference Kim, Jung and Yu10, Reference Testa, Biasi and Poli248) Thus, enhancing the supply of exogenous antioxidants might counteract the process of ageing and increase lifespan similar to ER. However, even though lipoic acid is a potent antioxidant, there are only a few studies showing increased lifespan in lipoic acid-supplemented invertebrates (C. elegans, D. melanogaster)(Reference Bauer, Goupil and Garber318–Reference Benedetti, Foster and Vantipalli320). While experiments in rodents did not find longevity-promoting effects under physiological conditions (in healthy animals with unrestricted food supply)(Reference Merry, Kirk and Goyns12, Reference Lee, Pugh and Klopp92, Reference Farr, Price and Banks321) immunosuppressed mice receiving a lipoic acid-supplemented diet revealed longer lifespans than controls(Reference Freisleben, Neeb and Lehr322). Another experiment showed that mice that were re-fed ad libitum after being on ER lived longer when the ad libitum feed was supplemented with lipoic acid compared with non-supplemented ad libitum feed-receiving mice(Reference Merry, Kirk and Goyns12).
Although evidence for lifespan-enhancing properties in rodents is missing, there are several publications pointing to the notion that lipoic acid counteracts age-related disorders. Lipoic acid was shown to reduce oxidative stress and damage in the heart muscle and brain in rats, thereby potentially ameliorating cardiac and neurodegenerative diseases(Reference Lee, Pugh and Klopp92, Reference Arivazhagan, Juliet and Panneerselvam323–Reference Liu, Killilea and Ames326).
Apart from directly scavenging ROS and recycling of other antioxidants like vitamin C or glutathione(Reference Packer, Witt and Tritschler315), lipoic acid might also decrease oxidative damage to macromolecules by decreasing the amount of ROS that is produced within the mitochondrial electron chain(Reference Dicter, Madar and Tirosh327). This protective effect may be mediated by enhancing the expression of UCP, which use the electrochemical gradient at the inner mitochondrial membrane to generate heat instead of ATP(Reference Bayir and Kagan328). Fatty acids are potent inductors of mitochondrial UCP(Reference Nedergaard, Golozoubova and Matthias329, Reference Shabalina, Jacobsson and Cannon330). It is assumed that lipoic acid might induce uncoupling due to its structural similarity to fatty acids(Reference Dicter, Madar and Tirosh327).
The controversial outcome of studies on lipoic acid regarding lifespan could be in part explained by the low stability of lipoic acid. Encapsulating lipoic acid in a cyclodextrin cavity increased its solubility(Reference Takahashi, Bungo and Mikuni331) and stability(Reference Takahashi, Bungo and Mikuni331, Reference Ikuta, Sugiyama and Shimosegawa332). Interestingly only this complexed form of lipoic acid increased energy expenditure in old mice. Most probably this was mediated by the induction of UCP(Reference Nikolai, Huebbe and Metges333). Importantly, a positive correlation between the metabolic rate, mitochondrial uncoupling and the lifespan of mice was reported(Reference Speakman, Talbot and Selman334). However, clear evidence for a positive relationship between lipoic acid intake and lifespan in healthy mammals is still lacking.
For a brief summary of the suggested molecular targets of the ERM candidates reviewed in this paper, see Fig. 9.

Fig. 9 Schematic overview of the suggested molecular targets of the energy restriction mimetic candidate substances 2-deoxy-d-glucose (2DG), metformin (MET), rapamycin (RAP), resveratrol (RSV), spermidine (SPD) and lipoic acid (LA). 2DG inhibits the central process of glycolysis, thereby favouring the activities of AMP-activated protein kinase (AMPK) and sirtuin (SIRT). MET increases AMPK activity, indirectly leading to increased autophagy and mitochondrial turnover. RAP inhibits mammalian target of rapamycin (mTOR) signalling, thereby favouring autophagy and inhibiting proliferative processes. In addition to its antioxidant capacity, RSV is thought to increase SIRT and AMPK activity. SPD might up-regulate the antioxidant response, enhance autophagy and decrease proliferation. LA might improve mitochondrial function, increase energy expenditure and reduce oxidative stress. ROS, reactive oxygen species.
Conclusion
Despite various benefits of ER, such as a reduced risk for age-related chronic diseases, the limitations of ER need to be considered. In order to prevent adverse side effects like immunosuppression or loss of bone mineral density, ERM should only imitate the positive effects. However, until now no substances have been found that repeatedly mimic the positive effects seen in restrictively fed models without adverse effects for mammals. In order to possibly improve the health-promoting effects or reduce negative side effects of single substances, ERM candidates should be tested for putative synergistic interactions. Moreover, it needs to be ascertained if other components found in Mediterranean or Asian (‘MediterrAsian’(Reference Pallauf, Giller and Huebbe335)) diets might exert ER-like effects similar to RSV and SPD. There are only a few studies that have investigated ER effects in humans, since lifespan studies in human subjects are difficult to perform (at the very least because of the length of human life). However, by means of measuring longevity biomarkers that have already been established in rodent or primate ER lifespan studies, putative healthspan- and lifespan-promoting effects in humans may be extrapolated. But due to the lack of feasibility of ER and the limitations of ERM candidate substances, alternative ER patterns should also be considered in future studies since there is strong evidence that moderate restriction regimens may improve healthspan(Reference Johnson, Laub and John220–Reference Mager, Wan and Brown223). Thus, more research is needed in order to find new dietary strategies that imitate positive ER effects without its downsides.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors contributed to the present review as follows: conception and design of the work (S. N., G. R., K. P. and P.H.); acquisition, analysis or interpretation of data (S. N. and K. P.); drafting of the manuscript (S. N. and K. P.); revising work critically for important intellectual content (K. P., G. R. and P.H.); and approval of the final version (S. N., K. P., P. H. and G. R.).
There are no conflicts of interest.