Introduction
The importance of the intestinal microbiota for gastrointestinal (GI) functions and health has been shown in many studies with human subjects, but also with model animals such as mice and pigs(Reference Turnbaugh, Ley and Mahowald1, Reference Guo, Xia and Tang2). In addition, several disease patterns in humans may be associated with the composition and/or metabolic activity of the intestinal microbiota. Relationships between variations in the abundance or metabolic activity of certain phyla and bacterial groups and the development of several medical conditions, such as obesity, have been established(Reference Turnbaugh, Ley and Mahowald1, Reference Ley, Backhed and Turnbaugh3). Other diseases related to changes in the composition and activity of the intestinal microbiota include diarrhoea and necrotising enterocolitis (NEC)(Reference Schwiertz, Gruhl and Löbnitz4, Reference Magne, Hachelaf and Suau5). As diet composition reflects the substrates available for the intestinal microbiota, thereby affecting their composition and metabolic activity, dietary modulation appears to be a valuable and promising tool to improve host health by beneficially steering microbial composition and metabolism(Reference Govers, Gannon and Dunshea6, Reference Duncan, Lobley and Holtrop7). Therefore, dietary supplementation of food additives, such as probiotics, has been frequently proposed. Probiotic food supplements, i.e. viable micro-organisms, may alter the microbiota of the host, thus beneficially influencing its health(Reference Schrezenmeir and de Vrese8), with Lactobacillus and Bifidobacterium species being among frequently used probiotics(Reference Marik9). Additionally, non-digestible food ingredients such as oligosaccharides can be used as prebiotics to modulate the gut microbiota, as they have proven to stimulate the growth and/or activity of beneficial bacterial groups in the colon(Reference Gibson and Roberfroid10).
In the past, rodents have been used most frequently as animal models. However, despite some advantages, such as their low costs in breeding, feeding and handling, several physiological and metabolic differences compared with humans have to be acknowledged. These differences include rodents being originally granivore animals in contrast to omnivorous humans, with fermentation taking place in their large caecum, while also practicing caecotrophy(Reference Houpt, Houpt and Pond11, Reference Graham and Aman12). Furthermore, the rat as a small animal needs more feed per unit body weight (BW), which means a faster digesta passage rate and, in addition, often a lower capacity for fibre digestion compared with humans(Reference Van Soest, Jeraci, Fosse, Wallace and Bell13). With respect to gut microbiota, the main bacterial groups such as Firmicutes and Bacteroidetes present in the faecal and caecal contents of rats and mice are similar compared with humans(Reference Brooks, McAllister and Sandoz14–Reference Tomas, Langella and Cherbuy16). However, the abundance of important bacteria genera such as Lactobacillus and Bifidobacterium spp. differs between humans and rats(Reference Lay, Rigottier-Gois and Holmstrom17–Reference Delroisse, Boulvin and Parmentier19).
The pig as a human-sized, omnivorous animal with anatomical and physiological similarities to humans has been proposed as an alternative animal model for research into dietary modulation of the human gut microbiota(Reference Oli, Petschow and Buddington20, Reference Andersen, Mølbak and Thymann21). Similar to humans, the gut microbiota of pigs mainly consists of the Firmicutes and Bacteroidetes phyla(Reference Leser, Amernuvor and Jensen22). In this context, it has to be mentioned that the pig has already been used for a long time as an animal model for research into human nutrition and biomedicine(Reference Miller and Ullrey23), as it has been described in several reviews comparing pigs with other animal models(Reference Baker24). According to these authors, there are diverse areas of application for the pig model including amino acid metabolism, total parenteral nutrition, rotavirus infection, and bacterial and viral pneumonia. Puiman & Stoll(Reference Puiman and Stoll25) reviewed the use of animal models to study neonatal nutrition in humans. They concluded that neonatal mice are suitable for mechanistic and genomic research in postnatal nutrition and associated diseases, while the neonatal pig is a suitable model to investigate acute and chronic effects of parenteral and enteral nutrition on whole-body metabolism in addition to specific tissues.
In order to assess the suitability of the pig as an appropriate model animal, a systematic comparison of the gut microbiota of pigs and humans is inevitable. In the present review, anatomical and physiological similarities and differences between the GI tract (GIT) of pigs and humans will be described, with special focus on the composition and metabolic activities of the microbiota harboured in the GIT of these two species. Studies in which the pig was used as a model to assess the role of the intestinal microbiota in disease development will be reviewed and, in particular, the impact of diet composition on the intestinal microbial community and its metabolic activity will be evaluated to identify beneficial effects on host health and recovery.
Anatomy of the gastrointestinal tract and body constitution – similarities and differences between humans and pigs
The porcine and human intestinal tracts are very similar with respect to anatomical and physiological characteristics(Reference Miller and Ullrey23, Reference Kien, Ailabouni and Murray26, Reference Labib, Erb and Kraus27). This includes comparable digesta transit times(Reference Miller and Ullrey23) and analogous digestive and absorptive processes(Reference Graham and Aman12). Moreover, the minimum nutrient requirements of pigs are similar to recommended daily allowances of humans when expressed per kg of dietary DM(Reference Miller and Ullrey23). Additionally, when calculating the relationship between intestinal length and BW, intestinal length amounts to 0·1 m/kg BW, for both humans and pigs(Reference Emmans, Kyriazakis and Kyriazakis28). However, the two species differ in the total length of their intestinal tracts. In humans, the length of the small and large intestines is 5·5–7 m and 1·5 m, respectively, at maturity (33 years)(Reference Mochizuki and Makita29), whereas the corresponding values in pigs amount to 15–22 and 4–6 m, respectively, at an assumed maturity age of 3 years(Reference Miller and Ullrey23, Reference Van Rens and Van Der Lende30). Other anatomical differences include a more distinct separation between duodenum, jejunum and ileum in humans compared with pigs, and a different arrangement of the small and large intestines in the abdomen of the two species(Reference Patterson, Lei and Miller31).
When using pigs as an animal model for humans, the rapid growth and mature size of modern swine breeds (90–120 kg and 330–450 kg at the age of 6 months and at the adult stage, respectively) to obtain maximal performance have to be taken into account when comparing the two species(Reference Rispat, Slaoui and Weber32). With regard to their body size, mini-pigs, with an adult BW of only 70–120 kg, might be closer to humans, while also being easier to handle, though more expensive(Reference Guilloteau, Zabielski and Hammon33).
Intestinal microbiota of humans and pigs: microbial fermentation and composition
Both pigs and humans are colon fermenters, and they have a similar composition of the colonic microbiota(Reference Miller and Ullrey23). However, symbiotic micro-organisms harboured in the GIT play a relatively minor role in the de novo synthesis of nutrients such as amino acids and fatty acids compared with ruminants(Reference Guilloteau, Zabielski and Hammon33). Pigs exhibit significant caecal fermentation(Reference Ehle, Jeraci and Robertson34), and may obtain up to 30 % of their energy requirement for maintenance from microbially produced SCFA in the large intestine(Reference Rérat, Fiszlewicz and Giusi35). On the contrary, humans lack a distinct caecum(Reference Argenzio and Stevens36), and only about 7 % of their energy requirement for maintenance originates from SCFA produced in the colon(Reference von Engelhardt, Bartels and Kirschberger37). In the GIT, SCFA and various gases (H2, CO2) are the major metabolites produced by microbial fermentation(Reference Allison and Macfarlane38). The largest fraction of SCFA is acetate, propionate and butyrate, with acetate being the most prominent of the three major SCFA, making up approximately two-thirds of the total SCFA(Reference Cummings and Englyst39). Acetate is extensively produced by various bacterial groups, while propionate and butyrate, which are known for their beneficial effects on the host(Reference Louis, Scott and Duncan40), are produced by a limited number of bacterial groups only. For example, propionate is largely metabolised in the liver where it is used as a precursor for gluconeogenesis and may inhibit lipogenesis(Reference Wright, Anderson and Bridges41, Reference Vogt, Pencharz and Wolever42), while butyrate is the preferred energy source for the colonic epithelial cells(Reference Pryde, Duncan and Hold43). Common butyrate producers in the GIT are clostridia such as Roseburia spp. and Eubacterium rectale (Reference Aminov, Walker and Duncan44). Increased concentrations of SCFA in the GIT, especially acetate, are suggested to secure a preventive effect on the overgrowth of endogenous Escherichia coli (Reference Asahara, Shimizu and Nomoto45).
The human gut microbiota: a brief overview
The composition of the intestinal microbiota in humans is extremely complex and therefore difficult to describe, and there exist plenty of data. The following section aims to provide a rather general and brief overview, thereby focusing on the major genera.
The human GIT contains about 10Reference Brooks, McAllister and Sandoz14 bacterial cells, with the highest density and diversity present in the large intestine(Reference Zoetendal, Akkermans and de Vos46, Reference Zoetendal, von Wright and Vilpponen-Salmela47). These bacteria belong to the Firmicutes group (about 60 %), Bacteroidetes (about 15 %), followed by Actinobacteria (about 15 %), Verrucomicrobia (about 2 %), Proteobacteria (about 1 %) and Methanobacteriales (about 1 %)(Reference Zoetendal, Vaughan and de Vos48, Reference Zoetendal, Rajilic-Stojanovic and de Vos49). In humans, the phylum Firmicutes comprises species belonging to the genera Eubacterium, Clostridium, Ruminococcus and Butyrivibrio (Reference Sghir, Gramet and Suau50) with the Eubacterium rectale–Clostridium coccoides group being represented in large numbers of total bacteria, accounting for about 28 % of total bacteria in faecal samples (Table 1)(Reference Lay, Rigottier-Gois and Holmstrom17). Bacteroidetes are represented by genera related to Bacteroides, which are generally present in high numbers in the human gut microbiota, averaging 9 to 42 % of total bacteria(Reference Doré and Corthier51). Actinobacteria, the third most prevailing phylum in the human GIT, comprise the Collinsella–Atopobium group, with 0·3–3·7 % of total bacteria(Reference Harmsen, Raangs and He52, Reference Rigottier-Gois, Bourhis and Gramet53), and bifidobacteria. Bifidobacteria are known for their health-promoting properties(Reference Gibson and Roberfroid10), and are used as probiotic food ingredients(Reference Flint, Duncan and Scott54), such as Bifidobacterium bifidum and Bifidobacterium longum (Reference Playne55). They compose about 4 % of the human faecal microbiota(Reference Lay, Rigottier-Gois and Holmstrom17, Reference Rigottier-Gois, Bourhis and Gramet53).
Table 1 Proportions of bacteria in human faeces assessed by fluorescent in situ hybridisation combined with flow cytometry detection* (Mean values and standard deviations)

* Donors on a Western European diet.
† Data compiled according to Lay et al. (Reference Lay, Rigottier-Gois and Holmstrom17). Age of donors: 7–52 years (n 91).
‡ Data compiled according to Rigottier-Gois et al. (Reference Rigottier-Gois, Bourhis and Gramet53). Age of donors: 3–68 years (n 23).
§ Data compiled according to Lay et al. (Reference Lay, Sutren and Rochet56). Age of donors: 25–45 years (n 21).
According to the results of representative studies as outlined in Table 1, lactobacilli may be present in colonic or faecal contents of humans to a lower extent, with levels comprising about 2·0 % of total bacteria(Reference Lay, Rigottier-Gois and Holmstrom17, Reference Lay, Sutren and Rochet56), or less (0·2–1·0 %)(Reference Mueller, Saunier and Hanisch57). However, there exists a considerable variation between individuals as well(Reference Reuter58). Streptococci in the human microbiota occur in a similar range as lactobacilli, amounting to 0·4–1·6 % of total bacteria(Reference Lay, Rigottier-Gois and Holmstrom17, Reference Lay, Sutren and Rochet56, Reference Fallani, Young and Scott59).
The porcine gut microbiota
Similar to humans, the gut microbiota of pigs mainly consists of the Firmicutes and Bacteroidetes phyla(Reference Leser, Amernuvor and Jensen22). The main bacterial groups in the pig GIT comprise the following bacteria: Streptococcus spp., Lactobacillus spp., Eubacterium spp., Fusobacterium spp., Bacteroides spp., Peptostreptococcus spp., Bifidobacterium spp., Selenomonas spp., Clostridium spp., Butyrivibrio spp., Escherichia spp., Prevotella and Ruminococcus spp.(Reference Leser, Amernuvor and Jensen22, Reference Moore, Moore and Cato60–Reference Hopwood, Hampson, Pluske, Le Dividich and Verstegen65). Kim et al. (Reference Kim, Borewicz and White66) assessed the pig's microbiota in faecal samples, which were collected five times in 3-week intervals starting at the age of 10 weeks. According to their results, the two most abundant bacterial genera of the pigs are Prevotella spp. (11·6 % of total bacteria), belonging to the Bacteroidetes phylum, and Anaerobacter (10·4 %), members of the Firmicutes. Interestingly, the abundance of Prevotella spp. decreased, while that of Anaerobacter spp. increased, with the age of the animal.
In contrast to the human GIT, the population of bifidobacteria present in the GIT of pigs is considerably lower, amounting to less than 1 % of total bacteria or being even undetectable(Reference Leser, Amernuvor and Jensen22). This has been confirmed in intestinal samples (stomach, small intestine, hindgut) of piglets collected at the age of 6 h to 20 d after birth; the results of this study are tabulated in Table 2(Reference Petri, Hill and Van Kessel67). Pieper et al. (Reference Pieper, Janczyk and Zeyner68) analysed the gut bacteria in the small intestine of piglets at 1, 2, 5 and 11 d post-weaning and did not detect any bifidobacteria and Escherichia coli on day 11 post-weaning. In addition, Enterobacteriaceae and members of the Clostridium coccoides–Eubacterium rectale cluster were only found occasionally. According to a study of Loh et al. (Reference Loh, Eberhard and Brunner69), bifidobacteria could be detected in the jejunum, ileum, and in colon samples in less than 40 % of the tested piglets. If they were present, Bifidobacterium spp. in the porcine intestine, such as Bifidobacterium suis, Bifidobacterium globosum or Bifidobacterium pseudolongum, differed from those generally found in the human GIT(Reference Mikkelsen, Bendixen and Jakobsen70, Reference Simpson, Stanton and Fitzgerald71). Nevertheless, the most abundant phylotypes of the pig are the lactic acid-producing bacteria, in particular lactobacilli(Reference Leser, Amernuvor and Jensen22), in contrast to humans, yet there exists a considerable variation of Lactobacillus spp. numbers as influenced by the age of the host animal(Reference Kim, Borewicz and White66). For example, in pigs at 10 weeks of age, lactobacilli averaged 11·0 % of total bacteria, whereas in pigs at 22 weeks of age their content decreased to 3·2 %(Reference Kim, Borewicz and White66), as shown in Table 3. In newborn piglets, 1·5 % of sequence samples could be assigned to Lactobacillaceae in the first hours of life, while their numbers increased to 44·6 % at day 20(Reference Petri, Hill and Van Kessel67). According to Krause et al. (Reference Krause, Easter and White72), who analysed Lactobacillus species in the pars oesophagus, ileum and caecum of weanling piglets, distribution of Lactobacillus in the GIT is also greatly influenced by the diet. In their study, relative abundance data indicated that L. brevis, L. fermentum and L. oris were by far the most abundant taxa. Also, L. plantarum was one of the predominant species isolated; however, it was only found in the pre-weaning period. According to Pieper et al. (Reference Pieper, Janczyk and Zeyner68), L. sobrius/L. amylovorus became dominant species in the small intestine of piglets from day 1 to day 11 post-weaning, whereas the abundance of L. salivarius and L. gasseri/johnsonii declined.
Table 2 Five most abundant bacteria families in piglets (n 6)* (adapted from Petri et al. (Reference Petri, Hill and Van Kessel67))

* Data for libraries prepared from three gastrointestinal tract locations (stomach, small intestine and hindgut) of piglets (6 h–20 d). Digesta collection at 6 h, 12 h, and at days 1, 2, 3, 5, 10 and 20.
Table 3 Five most abundant bacteria in pig faeces* and proportions of Roseburia, Clostridium, Bifidobacterium and Bacteroides (n 10) (adapted from Kim et al. (Reference Kim, Borewicz and White66))

* Pigs (age 10–22 weeks) on a commercial diet based on maize and soyabean meal.
† Collection of samples: weeks 10, 13, 16, 19 and 22. The average Shannon–Weaver and Simpson index values per group were 5.74 (sd 0·35) and 0·97 (sd 0·02) for farm (trial) 1, and 6·17 (sd 0·18) and 0·98 (sd 0·01) for farm (trial) 2.
Compared with humans, the abundance of streptococci in faeces is higher in pigs, averaging 7·4 % of total bacteria(Reference Kim, Borewicz and White66), with increasing numbers in the first days of life(Reference Petri, Hill and Van Kessel67). Moreover, the abundance of Bacteroides spp. in faecal samples appears to be much lower in the pig (0·1 % on average), in contrast to humans, where they belong to the frequently occurring bacteria(Reference Doré and Corthier51, Reference Kim, Borewicz and White66). On the other hand, according to Guo et al. (Reference Guo, Xia and Tang2), Bacteroides spp. averaged 3·8 % of total bacteria in faeces of 5-year-old (lean) Banna mini-pigs.
Main determinants affecting intestinal microbiota composition
Principally, composition of the gut microbiota depends on a variety of exogenous factors. Diet, age and environmental conditions are important determinants(Reference Leser, Lindecrona and Jensen73, Reference Mariat, Firmesse and Levenez74), while in pigs sanitary conditions and coprophagy also play a significant role. Furthermore, the host's immune system exerts major impacts on the microbial ecosystem, which has been reviewed elsewhere (for example, Hooper et al. (Reference Hooper, Littman and Macpherson75)).
Environment
At birth, the intestines of infants are sterile(Reference Bezirtzoglou76); however, within a few hours, bacteria are detectable in faeces. Initially, these are facultative aerobes; thereafter, through consumption of oxygen by these bacteria, follows colonisation with strict anaerobes(Reference Bezirtzoglou76). These bacteria mainly originate from the mother and the environment, with the mode of delivery being the major determinant of the composition of the intestinal microbiota. While vaginally born infants are colonised first by faecal and vaginal bacteria of the mother, caesarean section leads to colonisation with bacteria from the hospital environment and health care workers(Reference Bezirtzoglou76, Reference Gronlund, Lehtonen and Eerola77). According to a study of Penders et al. (Reference Penders, Thijs and Vink78), caesarean section resulted in lower colonisation rates and counts of bifidobacteria and higher Escherichia coli counts, while hospitalisation was associated with higher colonisation rates of Clostridium difficile. In pigs Clostridium difficile is well adapted to the intestinal tract irrespective of the environmental conditions, which means that no differences could be observed in intestinal Clostridium numbers when comparing an indoor- with an outdoor production system(Reference Susick, Putnam and Bermudez79).
In infants, the intestinal microbiota has been found to be influenced by the environment during birth, prematurity, hygiene measures, and the type of infant feeding(Reference Heavey and Rowland80). In this context, the number of siblings has been suggested to play a role, since a greater proportion of bifidobacteria was found in infants with older siblings compared with those without siblings(Reference Penders, Thijs and Vink78). On the other hand, a relationship between the composition of the gut microbiota and the presence of furry pets or farm residence could not be shown(Reference Penders, Thijs and Vink78).
Nutrition
The high impact of nutrition on the microbiota in neonates is obvious since earlier colonisation with bifidobacteria has been shown in breast-fed infants compared with in infants fed with formula(Reference Yoshioka, Iseki and Fujita81). This is probably due to the presence of oligosaccharides in human milk that exhibit growth-promoting effects on bifidobacteria(Reference Kunz and Rudloff82, Reference Newburg83). In Table 4, proportions of bacteria in infant faecal samples are summarised, with the Bifidobacterium genus accounting for 40–75 % of the total detectable bacteria(Reference Leser, Amernuvor and Jensen22, Reference Langendijk, Schut and Jansen84). The milk itself contains bacteria as well, with an estimated intake of 1 × 105 to 1 × 107 bacteria, based on daily consumption of 800 ml milk(Reference Heikkilä and Saris85). In particular, staphylococci and streptococci have been detected, with Staphylococcus epidermidis, Streptococcus salivarius and Streptococcus mitis being most prevailing(Reference Heikkilä and Saris85, Reference West, Hewitt and Murphy86), although maternal skin contact while breast-feeding could have been responsible for their prevalence as well(Reference West, Hewitt and Murphy86). As these bacteria have also been documented in stool samples of breast-fed infants(Reference Kirjavainen, Apostolou and Arvola87, Reference Favier, Vaughan and De Vos88), it can be speculated that the bacterial composition of breast milk reflects infant faecal microbiota. In pigs, the bacterial community of the GIT also adapts to changes in the animal's diet, as has been observed following the feeding of different experimental diets(Reference Leser, Lindecrona and Jensen73); diet has an influence on the distribution of the microbiota in the GIT too, as described by Krause et al. (Reference Krause, Easter and White72) for lactobacilli.
Table 4 Proportions of bacteria in infant faecal samples (age 6 weeks) from five European Union countries assessed by fluorescent in situ hybridisation combined with flow cytometry (n 606) (adapted from Fallani et al. (Reference Fallani, Young and Scott59)) (Mean values and standard deviations)

Age
The impact of age on gut microbiota composition has been observed by Mariat et al. (Reference Mariat, Firmesse and Levenez74), who confirmed the dominance of bifidobacteria in the microbiota of infants. In addition, higher proportions of lactobacilli in infants compared with seniors, and lower percentages of Clostridium leptum and Clostridium coccoides in infants than in adults, have been reported by Mariat et al. (Reference Mariat, Firmesse and Levenez74). Moreover, these authors determined an increase in Escherichia coli in seniors compared with adults. In pigs, differences in bacterial numbers as influenced by progressing age were obtained as well. According to Petri et al. (Reference Petri, Hill and Van Kessel67), Clostridiaceae accounted for 34 % of total sequences in piglets 6 h after birth, while only 1 % was found at week 22 of age (Table 2)(Reference Petri, Hill and Van Kessel67). In the same study, Enterobacteriaceae could not be detected at day 20 of age. In another study(Reference Pieper, Janczyk and Schumann89), however, Enterobacteriaceae counts were in a steady state from the day of weaning (28 d of age) until day 5 post-weaning, whereas significantly lower counts were found on day 11 post-weaning. Similarly, in 10-week-old pigs, 26 % on average of total sequences in faeces could be assigned to Prevotella spp., while in week 22, abundance decreased to about 4 % (Table 3)(Reference Kim, Borewicz and White66).
Sanitary conditions and coprophagy
In piglets, the influence of sanitary conditions on gut microbiota composition has been established(Reference Montagne, Arturo-Schaan and Le Floc'h90). Faeces of piglets raised under poor sanitary conditions (facilities not disinfected or cleaned after previous occupancy with pigs from the same herd) compared with faeces of animals kept in a clean environment contained significantly more Lactobacillus spp. and enterobacteria(Reference Montagne, Arturo-Schaan and Le Floc'h90). In addition, fewer anaerobic sulfite-reducing bacteria were present, which are considered detrimental bacteria due to their production of hydrogen sulfide which, in turn, may damage the intestinal epithelium(Reference Montagne, Arturo-Schaan and Le Floc'h90). Furthermore, poor sanitary conditions may stimulate butyrate production(Reference Montagne, Arturo-Schaan and Le Floc'h90), which is assumed to be beneficial for the host(Reference Thangaraju, Cresci and Liu91). Most likely, butyrate production can be attributed to the presence of lactobacilli, since these bacteria produce lactate, which, in turn, is utilised by butyrate-producing bacteria(Reference Duncan, Louis and Flint92).
Coprophagy has been observed by Watson & Bertram(Reference Watson and Bertram93) in intensively reared sows, and Gleed & Sansom(Reference Gleed and Sansom94) reported the consumption of faeces by piglets through behaviour such as suckling an udder covered with faeces and rubbing littermate's bodies. This might explain to a certain degree existing differences in the composition of the microbiota between pigs and humans, since environmental conditions and resultant behaviour patterns between pigs and humans differ significantly. Nevertheless, principal differences between the gut microbiota of humans and pigs have to be considered as well. Schmidt et al. (Reference Schmidt, Mulder and Musk95) found Streptococcus spp. and diverse Lactobacillus strains (L. reuteri, L. amylovorous, L. johnsonii, L. brevis, L. pentosus and L. plantarum) in the ileum of isolator-reared piglets. Of total clones, piglets transferred to the isolator 2 d after birth from an indoor rearing facility contained 27·4 % Lactobacillaceae-affiliated clones(Reference Schmidt, Mulder and Musk95), which in this case cannot be attributed to pig-specific environmental conditions or behaviour patterns.
Role of the intestinal microbiota in host health and disease development and its dietary modulation in studies with pigs
The use of pigs as a relevant human medical model is well documented(Reference Lunney96). Also, pigs and humans share similarities in GI microbial diversity(Reference Lamendella, Domingo and Ghosh97). In comparison with other animal models including rodents, pigs allow for more invasive sampling in the GIT, induction of disease states, and a variety of nutritional intervention approaches. Thus, several studies have used the pig model when investigating disease states related to the intestinal microbiota, and their possible modulation by dietary means. These include studies on weanling diarrhoea, first of all, since piglets naturally are susceptible to weanling diarrhoea similar to human infants, and, second, since pigs may be used as suitable animal models when experimentally inducing secretory diarrhoea to assess possible effects of feed/food additives such as pre- or probiotics. Due to the development of very similar symptoms and course of disease in both infants and piglets, studies on the dietary treatment of NEC will be considered in the following review as well. Finally, in line with recent concern on the possible relationship between intestinal microbiota and the development of obesity, the present review will also focus on studies on this chronic disease, thereby revealing possibilities to modulate the gut microbiota beneficially. As for the latter, while studies have mainly been performed with rodent models, the alternative use of the pig will be reviewed.
Diarrhoea: occurrence after weaning in human infants and piglets
For infants, the time of introduction of food other than breast milk is a high-risk period due to the occurrence of diarrhoeal diseases, which represent a main health problem worldwide, affecting primarily neonates and children(Reference Nabuurs98, Reference Fujiwara, Hashiba and Hirota99). This so-called ‘weanling diarrhoea’(Reference Gordon, Chitkara and Wyon100) is accompanied by shifts in the composition of faecal microbiota(Reference Magne, Hachelaf and Suau5), indicating changes in the intestinal microbiota of the infant due to dietary modifications. As a result, an increased susceptibility to infectious diseases has been observed(Reference Nabuurs, van Zijderveld and de Leeuw101). It has been shown that 10 to 30 % of cases of sporadic endemic infant diarrhoea occurred following an enterotoxigenic Escherichia coli infection(Reference Nataro and Kaper102), producing enterotoxins and attaching to enterocytes(Reference Gaastra and de Graaf103). On the other hand, feeding breast milk protects against diarrhoea, firstly by minimising the infant's exposure to contaminated foods and fluids(Reference de Zoysa, Rea and Martines104). Second, it may reduce the incidence and severity of infections in the infant by synergistic actions of several bioactive molecules present in colostrum and milk, including immunocompetent cells, immunoglobulins, fatty acids, polyamines, oligosaccharides, lysozyme, lactoferrin and other glycoproteins, as well as antimicrobial peptides(Reference Newburg105). Furthermore, mother's milk may be considered as a source of potentially beneficial bacteria, such as certain Lactobacillus spp., protecting mothers and/or infants against a variety of allergic, inflammatory and infectious diseases(Reference Lara-Villoslada, Olivares and Sierra106).
For piglets, the weaning transition is a complex period during which they have to cope with abrupt separation from their mother, mixing with other litters in a new environment, and switching from highly digestible milk to less digestible, more-complex solid feed. Sows' milk, similar to human milk, provides several protecting factors, including maternal cells such as phagocytes, lymphocytes and epithelial cells, as well as antimicrobial substances, for example, lactoferrin and lysozyme(Reference Wagstrom, Yoon and Zimmerman107). Additionally, the probiotic potential of certain bacterial species in the milk, such as L. reuteri, has been demonstrated(Reference Martín, Delgado and Maldonado108). Consequently, weaning means the withdrawal of these defensive factors in the milk, while the weaning period is generally accompanied by morphological, histological and microbial changes in the GIT of young animals(Reference Cummins, Steele and LaBrooy109, Reference Pluske, Hampson and Williams110). Enteropathogenic bacteria and their interactions in the small intestine represent an additional burden for the newly weaned piglet(Reference Pluske, Hampson and Williams110). Studies with piglets have shown that not only haemolytic enteropathogenic Escherichia coli, but also rotavirus, one of the major viral agents accountable for the development of diarrhoea, are responsible for the outbreak of this disease(Reference Lecce, Clare and Balsbaugh111). In humans, rotavirus is the most important reason for diarrhoea hospitalisation among children(Reference Parashar, Bresee and Gentsch112), and causes 440 000 deaths annually in children below 5 years of age worldwide(Reference Parashar, Gibson and Bresee113). Lecce et al. (Reference Lecce, Clare and Balsbaugh111) concluded in their study with piglets that by damaging the epithelium of the small intestine, which is frequently associated with malabsorption, this virus creates the required environment for the subsequent colonisation and growth of Escherichia coli. However, it has to be considered that the severity and localisation of rotavirus infections may vary among animal species and between studies. Moreover, some rotavirus infections are asymptomatic, which suggests that both viral and host factors can affect disease severity(Reference Ramig114).
Dietary strategies as therapeutic tools for diarrhoeal diseases
Several dietary tools targeting the intestinal microbiota, thereby acting as a prophylactic option in the prevention of diarrhoea, are currently under investigation, including probiotics and prebiotics(Reference Shu, Freeman and Harsharnjit115, Reference Gill and Guarner116). While multiple studies have been performed investigating the effect of feed additives, such as probiotics, on weanling diarrhoea in piglets with the aim of improving production conditions(Reference Lallès, Bosi and Smidt117), the effect of specific food additives in alleviating diarrhoea has been assessed using human subjects as well(Reference Szajewska and Mrukowicz118). In addition, pigs have also been used to examine the potential of these supplements to influence human health beneficially(Reference Shu, Freeman and Harsharnjit115).
As a possible mechanism for probiotics to alleviate or prevent diarrhoea, enhancing immune responses has been suggested. For example, immune function-enhancing effects have been shown for the probiotic Bifidobacterium lactis HN019 in studies with mice(Reference Gill, Rutherford and Prasad119) and human subjects(Reference Arunachalam, Gill and Chandra120). Accordingly, in a study using a piglet model of weanling diarrhoea(Reference Shu, Freeman and Harsharnjit115), animals that received Bifidobacterium lactis HN019 showed lower concentrations of faecal rotavirus and Escherichia coli, higher blood leucocyte phagocytic and T-lymphocyte proliferate responses, and higher GIT pathogen-specific antibody titres. These piglets also showed a lower severity of weanling diarrhoea and an improved feed conversion ratio during weaning, compared with control piglets not receiving the probiotic. The authors assumed a mechanism of enhanced immune-mediated protection, and suggested similar beneficial effects for the probiotic use in infants(Reference Shu, Freeman and Harsharnjit115).
The health-promoting effect of probiotics was also confirmed in a study using a pig model of secretory diarrhoea(Reference Schroeder, Duncker and Barth121), i.e. diarrhoea produced by an increase in colonic secretions(Reference Eisenberg122). Here, the dietary supplementation of the probiotic strain Escherichia coli Nissle 1917 exhibited protective effects against diarrhoea caused by the toxigenic Escherichia coli strain Abbotstown(Reference Schroeder, Duncker and Barth121). This strain was administered via an orogastric tube to establish a pig model of secretory diarrhoea(Reference Schroeder, Duncker and Barth121). Along with the clinical signs of diarrhoea, jejunal epithelia tissues of animals that did not receive the probiotic showed an increased secretory response after stimulation of the cyclic AMP-mediated second messenger pathway by forskolin. This indicates an increased excitability of chloride secretory systems under infected conditions(Reference Schroeder, Duncker and Barth121). In Ussing chamber experiments, forskolin is often used with intestinal tissues from different species including the pig to induce a secretory response, specifically via cyclic AMP-mediated Cl– secretion(Reference Brown, Overend and Treder123–Reference Cermak, Follmer and Wolffram125). Pretreatment with the probiotic strain Escherichia coli Nissle 1917 completely abolished clinical signs of secretory diarrhoea, and the jejunum epithelia of these animals did not exhibit an increased secretory response upon stimulation with forskolin(Reference Schroeder, Duncker and Barth121).
Furthermore, the use of prebiotics has been suggested to improve host health by beneficially influencing the composition and metabolic activity of the gut microbiota. In this context, the beneficial effects of inulin on the gut microbiota in human subjects have been described, particularly by enhancing colonic bifidobacteria numbers, although results may vary probably due to variations in chain length and dosage of the type of inulin used(Reference Gibson and Roberfroid10). For example, in a study of Patterson et al. (Reference Patterson, Yasuda and Welch126) with pigs, a short-chain inulin product was already partly fermented in the jejunum and ileum. In contrast, long-chain inulin was not degraded until reaching the distal ileum or the caecum(Reference Patterson, Yasuda and Welch126).
Increased numbers of bifidobacteria have been detected upon dietary supplementation with prebiotic fructo-oligosaccharides (FOS) in studies with human subjects(Reference Gibson and Roberfroid10, Reference Buddington, Williams and Chen127), while infant formula supplemented with a mixture of galacto-oligosaccharides and FOS resulted in higher faecal counts of bifidobacteria and lactobacilli compared with infants fed unsupplemented formula(Reference Penders, Thijs and Vink78). According to Donovan et al. (Reference Donovan, Wang and Li128), human milk oligosaccharides may be responsible for the differences in development, microbiota and incidence of disease between breast-fed and formula-fed infants, due to their abundance and diversity, large physiological actions and absence in infant formula. Polydextrose has been proposed as a surrogate for human milk oligosaccharides, and displayed prebiotic properties in a study with suckling piglets by increasing ileal lactobacilli and propionic and lactic acid concentrations and decreasing pH with associated alterations in ileal cytokine expression(Reference Herfel, Jacobi and Lin129). Furthermore, its safety as a food additive has been assessed, with measurements of diverse morphological, histological and biochemical parameters indicating that the supplementation of formula with polydextrose between 1·7 g/l (1·0 g/kg BW per d) and 17 g/l (8·35 g/kg BW per d) does not show any toxicological effects on neonatal pigs, further supporting the safe use of this prebiotic carbohydrate in the nutrition of human neonates(Reference Herfel, Jacobi and Lin130).
In a pig model, FOS has been tested for its beneficial effects on secretory diarrhoea. Cholera toxin-inducing secretory diarrhoea was evaluated in 21-d-old pigs treated with FOS in combination with oral electrolyte solutions (OES)(Reference Oli, Petschow and Buddington20). In healthy piglets, supplemental OES in combination with FOS increased the numbers of lactobacilli in most parts of the GIT (colon, caecum, mid-small intestine), but mostly in the colon, with 50-fold increased counts relative to normal healthy pigs without supplementation(Reference Oli, Petschow and Buddington20). In piglets administered the diarrhoea-inducing cholera toxin, the addition of OES and FOS did not result in a reduction of diarrhoea and the associated loss of water. Thereafter, during the recovery phase from diarrhoea, piglets treated for 24 h with OES and FOS responded to this supplementation with higher proliferation rates of lactobacilli, especially in the colon. Compared with healthy piglets and recovering animals treated with OES solely, the supplementation of OES + FOS led to significantly higher densities of lactobacilli after 24 h in all samples (colon, caecum, mid-small intestine) of these piglets(Reference Oli, Petschow and Buddington20).
Furthermore, research concerning diarrhoea caused by carbohydrate malabsorption in patients during enteral feeding has been carried out using the pig as a model. Kien et al. (Reference Kien, Chang and Cooper131) applied inulin as a fermentable carbohydrate that does not cause osmotic diarrhoea before inducing diarrhoea in pigs following lactulose malabsorption. With regard to humans, administering inulin should potentially prevent diarrhoea caused by lactulose malabsorption following enteral feeding. In addition, inulin might attenuate some of the unfavourable effects of severe lactulose malabsorption(Reference Kien, Murray and Qualman132). Inulin, with an average of thirty-five fructosyl units, is supposed to show much less of an osmotic effect on the colon compared with lactulose(Reference Kien, Chang and Cooper131). By prefeeding the prebiotic inulin, diarrhoea caused by the application of lactulose could be relieved(Reference Kien, Chang and Cooper131). These findings are in agreement with observations of Flourie et al. (Reference Flourie, Briet and Florent133), who showed that primarily offering adult human subjects a low dose of lactulose increases fermentation and moderates diarrhoea during a period following a high dose of lactulose consumption. It appears that pre-feeding represents a form of adaption of the colonic microbiota, since a dose of indigestible carbohydrate that can be completely fermented might increase the overall capacity for fermentation, partly by inducing the production of bacterial glucosidases(Reference Flourie, Briet and Florent133).
In addition to pre- and probiotic applications, other feed additives might beneficially influence host health as related to diarrhoeal disease. Torrallardona et al. (Reference Torrallardona, Conde and Badiola134) evaluated spray-dried animal plasma as an alternative to antimicrobial medication with colistin sulfate in weanling pigs challenged with Escherichia coli K99. The performance response to spray-dried animal plasma was similar to that obtained with the antibiotic colistin; thus, spray-dried animal plasma may be a suitable alternative to the use of antibiotics. The length of the villi could be maintained, and a higher small-intestinal weight was observed following the application of both products. Furthermore, they also had a direct effect on the microbial population of the GIT, with plasma supplementation stimulating the growth of lactobacilli in the ileum and caecum(Reference Torrallardona, Conde and Badiola134). Other studies revealed positive effects of black tea extract (BTE) or green tea extract on GIT function of mice (ex vivo), calves and pigs(Reference Toda, Okubo and Ikagai135–Reference Bruins, Cermak and Kiers137), including beneficial effects of dietary green tea extract or polyphenols on the gut microbiota of both animals(Reference Terada, Hara and Nakajyo138–Reference Ishihara, Akachi, Yamamoto, Juneja, Chu and Kim140) and human subjects(Reference Hara141). Moreover, there is evidence that flavonoids from both black and green tea possess anti-microbial properties against several pathogenic bacteria including pathogenic strains of Escherichia coli (Reference Friedman142), by removing Fe from Fe-dependent pathogens including Escherichia coli (Reference Neilands143). Recently, the effects of BTE on the prevalence of diarrhoea in a model of enterotoxigenic Escherichia coli-infected post-weaning piglets were assessed(Reference Bruins, Vente-Spreeuwenberg and Smits144). In this study, dietary BTE supplementation decreased diarrhoea frequency in piglets by 20 % throughout an experimental period of 27 d. However, at the same time feed intake and feed efficiency were reduced by 16 and 12 %, respectively, as piglets preferred the control over the BTE-containing diets(Reference Bruins, Vente-Spreeuwenberg and Smits144), probably due to the presence of astringent theaflavins in tea(Reference Ngure, Wanyoko and Mahungu145). Since no correlation could be obtained between feed intake and the occurrence of diarrhoea, the lower incidence of diarrhoea was associated with BTE supplementation rather than with reduced feed intake(Reference Bruins, Vente-Spreeuwenberg and Smits144). In vitro, Bruins et al. (Reference Bruins, Vente-Spreeuwenberg and Smits144) observed a 24 h delay at least in the exponential growth of enterotoxigenic Escherichia coli cultures following the addition of BTE. In conclusion, despite the anti-nutritional properties of BTE, further studies are warranted to develop a suitable mode of application of BTE in the treatment of diarrhoea(Reference Bruins, Vente-Spreeuwenberg and Smits144).
Necrotising enterocolitis
The GI inflammatory disorder NEC represents one of the most serious diseases for preterm neonates. Early symptoms of this disease are abdominal distension, food intolerance, regurgitation and lethargy, in both human infants and piglets(Reference Neu and Weiss146, Reference Sangild147). Pathological changes in the intestinal wall occur mostly in the distal small intestine and colon of infants and piglets, resulting in the necrosis of the complete mucosa(Reference Neu148, Reference Travadi, Patole and Simmer149). Pneumatosis intestinalis is a further symptom of NEC in infants as well as in pigs, which has been described as an accumulation of gas produced by bacteria(Reference Lee and Polin150). Many factors play a role in disease progression in preterm neonates (human and pig) such as nutritional and immunological dysfunction as well as bacterial colonisation(Reference Siggers, Siggers and Thymann151). However, potential interactions among these variables remain unclear. The immature intestine conditions may result in accumulated undigested food, eventually resulting in bacterial overgrowth and exaggerated fermentation(Reference Siggers, Siggers and Thymann151). In this context, the abundance of Escherichia coli, Klebsiella (duodenal aspirates) and Clostridium spp. (stool samples) is often assumed to be associated with the incidence of NEC in infants(Reference Hoy, Wood and Hawkey152, Reference de la Cochetiere, Piloquet and des Robert153). Studies with germ-free pigs revealed that NEC does not occur in the absence of bacteria(Reference Sangild147, Reference Jiang, Sangild and Siggers154). Principally, the application of broad-spectrum antibiotics in caesarean-delivered preterm pigs, administered together with formula, could prevent the development of NEC; however, several species, which are commonly associated with human infections, can survive the antibiotic treatment, and overgrowth of these species could potentially result in intestinal inflammation and necrosis(Reference Cilieborg, Boye and Molbak155). Therefore, broad-spectrum antibiotics may prevent NEC in the short term, but may lead to an increased risk of NEC in the long term due to overgrowth of pathogens(Reference Cilieborg, Boye and Molbak155). In infants and pigs, especially the occurrence of Clostridium perfringens seems to be associated with an increased frequency of NEC(Reference Sangild147, Reference de la Cochetiere, Piloquet and des Robert153). Nevertheless, inducing NEC by the inoculation of preterm pigs with Clostridium perfringens type A failed(Reference Cilieborg, Boye and Molbak155), and, in addition, Clostridium perfringens type C and D toxin immunisation of the preterm piglets did not protect against NEC in this study(Reference Cilieborg, Boye and Molbak155). Thus, the presence of Clostridium perfringens in NEC may be a response to disease rather than a cause(Reference Cilieborg, Boye and Molbak155).
In comparison with the pig, in humans the functional maturation of the GIT starts early after birth, but progresses slowly over time(Reference Sangild, Siggers and Schmidt156). Therefore, the newborn is able to digest significant quantities of non-milk carbohydrates and proteins additionally to nutrients contained in milk(Reference Sangild, Siggers and Schmidt156). In contrast, in the pig, functional development of the gut takes place both pre- and postnatally(Reference Sangild, Siggers and Schmidt156), resulting in a less developed GIT at birth when compared with human infants. At peak lactation of the mother, the gut capacity of piglets is about twice as high as that of the human infant, though both show similar BW(Reference Darragh and Moughan157). Nevertheless, the advantage of the preterm piglet as a model animal to study NEC symptoms results from the very similar clinical and histological characteristics of this syndrome compared with the infant, together with an analogous development of the symptoms(Reference Bjornvad, Thymann and Deutz158). Furthermore, the possibility to apply ‘total parenteral nutrition’ in neonatal piglets is of further advantage, since it is considered to promote intestinal sickness and NEC in preterm infants(Reference Siggers, Siggers and Boye159).
Dietary strategies as therapeutic tools for necrotising enterocolitis
The high impact of nutrition and environment on the intestinal microbiota of preterm infants is reflected in the microbial composition of stool samples of infants in their first week after birth: while formula-fed infants on day 6 after birth exhibit relatively high levels of enterobacteria, compared with lower numbers of bifidobacteria, in breast-fed infants bifidobacteria usually become dominant during the first week of life(Reference Yoshioka, Iseki and Fujita81). Additionally, preterm birth and formula feeding are associated with a predisposition for variations in the composition of the faecal microbiota and frequency of NEC compared with full-term neonates and infants receiving mother's milk(Reference Schwiertz, Gruhl and Löbnitz4, Reference Penders, Thijs and Vink78). Thus, it has been suggested that dietary supplementation with probiotics might be a preventive option against the development of NEC. Generally, an enhanced proliferation of beneficial members of the GIT microbial ecosystem, together with favourable effects on intestinal permeability, intensified reaction of the intestinal immune system, and increased production of anti-inflammatory cytokines, is associated with diverse probiotic modes of action(Reference Braegger and Bischoff160).
In a piglet model, a probiotic treatment consisting of a mixture of Bifidobacterium animalis and four Lactobacillus species (L. acidophilus, L. casei, L. pentosus and L. plantarum) reduced NEC severity and mucosal atrophy and disorder when being administered together with formula instantly after delivery(Reference Siggers, Siggers and Boye159). Moreover, growth of lactobacilli was significantly enhanced in the stomach, the small intestine and the colon, while the abundance of the potential pathogen Clostridium perfringens diminished significantly in the small intestine and colon(Reference Siggers, Siggers and Boye159). In the same study, proliferation of coliforms was slightly reduced, and enterococci decreased significantly in the stomach and small intestine. Principally, the growth of these bacteria is stimulated following formula feeding(Reference Schwiertz, Gruhl and Löbnitz4), and they have been identified as potential pathogens in human preterm neonates(Reference Westerbeek, van den Berg and Lafeber161, Reference Claud and Walker162). According to the study of Siggers et al. (Reference Siggers, Siggers and Boye159), piglets fed formula without the addition of probiotics showed a noticeable increase in the occurrence of NEC and severity of clinical symptoms. It has been suggested that octanoic acid might have been responsible for the higher incidence of stomach NEC in piglets fed with the formula diet, as only small amounts of this acid were present in the formula diet, but higher concentrations of octanoic acid were found in the stomach of the piglets(Reference Siggers, Siggers and Boye159). In contrast, in piglets fed with the formula diet supplemented with probiotics, significantly lower levels of gastric octanoic acid were determined. Obviously, octanoic acid represents a metabolite resulting from gastric digestion and fermentation of the medium-chain TAG fraction of the diet, with higher amounts possibly being responsible for the increased rate of NEC occurrence(Reference Lin163, Reference Peng, He and Chen164). It appears that the reduction of octanoic acid concentration in the gastric lumen as observed in the study of Siggers et al. (Reference Siggers, Siggers and Boye159) can be attributed to the dietary supplementation of probiotics, resulting in potential protection against NEC. This feature of probiotics is of special interest for the protection of the vulnerable small intestine and colon of preterm neonates, but more research is warranted to further elucidate these probiotic characteristics. Concerning medium-chain fatty acids (MCFA), it has also been shown that MCFA-containing fat sources and lipolytic enzymes could be a valuable alternative to nutritional antibiotics in piglets(Reference Dierick, Decuypere and Molly165). In the study of Dierick et al. (Reference Dierick, Decuypere and Molly165), the antimicrobial effects of MCFA from three selected MCFA-containing fat sources and one appropriate microbial lipase were investigated in an in vitro model, finding that a minimal concentration of 0·025 m-MCFA in the medium (for example, stomach, proximal gut) seems to be necessary to achieve a significant (>10-fold) bacterial suppression. Changes in microbial ecology due to the ingestion of MCFA have also been investigated by Zentek et al. (Reference Zentek, Buchheit-Renko and Manner166), who measured higher concentrations of caprylic (octanoic) and capric (decanoic) acid in the stomach of piglets fed MCFA diets, uncoated or coated with vegetable fat and lecithin, compared with a control group. Ingestion of MCFA diets led to an increase in the number of eubacteria, Enterobacteriaceae, clostridial clusters I and IV, L. johnsonii and L. amylovorus in gastric contents. Changes in concentrations of SCFA could also be observed, with lower levels of propionic, n-butyric and isovaleric acid and numerically higher concentrations of acetic acid in the small intestine. Ammonia concentrations increased in the distal small intestine of the MCFA groups. Obviously, MCFA can influence microbial ecology in the stomach and bacterial metabolites in the small intestine(Reference Zentek, Buchheit-Renko and Manner166).
Interestingly, the development of NEC is assumed to be linked to higher levels of SCFA in the premature human intestine(Reference Clark, Thompson and Weiner167), and the administration of SCFA can cause mucosal injury in rats(Reference Lin, Nafday and Chauvin168). Moreover, Cilieborg et al. (Reference Cilieborg, Boye and Molbak155) determined significantly higher concentrations of acetate in the stomach, and significantly more acetate and butyrate in the colon of piglets with NEC compared with healthy animals. Butyrate has been shown to increase mucosal injury by enhancing the production of stromelysin-1 in cytokine-stimulated gut mesenchymal cells(Reference Pender, Quinn and Sanderson169). Generally, high levels of clostridia have been linked with the production of butyric acid, while bifidobacteria, by causing a decrease in clostridia numbers, have been associated with a decrease or disappearance of butyric acid(Reference Butel, Roland and Hibert170). Furthermore, it has been reported that the application of probiotic bifidobacteria reduced both the incidence and severity of NEC in premature neonates(Reference Bin-Nun, Bromiker and Wilschanski171, Reference Lin, Su and Chen172).
The positive results on the incidence of NEC following the application of probiotics in pigs have been confirmed in human subjects(Reference Braegger and Bischoff160, Reference Lin, Su and Chen172, Reference Hoyos173). However, studies with preterm piglets also indicated that viable or inactivated probiotic strains (Bifidobacterium animalis, L. paracasei and Streptococcus thermophiles) increased the incidence and severity of NEC(Reference Cilieborg, Thymann and Siggers174). Moreover, bacteraemia and sepsis have been attributed to an applied Lactobacillus strain in a 6-week-old infant and a 6-year-old child(Reference Land, Rouster-Stevens and Woods175), and sepsis secondary to probiotic Bifidobacterium breve administration has been observed by Ohishi et al. (Reference Ohishi, Takahashi and Ito176). As preterm neonates are primarily colonised by bacteria of low diversity, and because their gut immune system is immature, hypersensitivity of the intestine to administration of probiotic bacteria may occur, which is consistent with increased bacterial translocation and neonatal mortality in immunodeficient mice after probiotic administration(Reference Cilieborg, Thymann and Siggers174, Reference Wagner, Warner and Roberts177). Thus, under certain conditions, some probiotic strains may be harmful for immunocompromised patients with disturbed gut function(Reference Cilieborg, Thymann and Siggers174).
Further studies have to be conducted before establishing routine probiotic supplementation to premature neonates, since factors such as the optimal dose, strain(s), timing and duration of administration, and side effects have not yet been sufficiently investigated(Reference Cilieborg, Boye and Sangild178). Also, previous studies have only focused on a few strains of Bifidobacterium, Lactobacillus and Streptococcus, suggesting the need for the assessment of other possible probiotic candidates(Reference Siggers, Siggers and Thymann151), as well as the application of a suitable animal model such as the pig.
Obesity
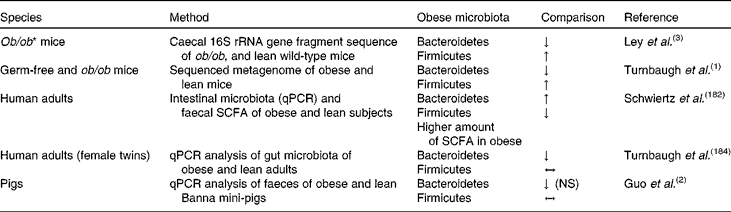
Nowadays, obesity has become one of the major health issues, with 1·4 billion overweight adults, 20 years and older, and almost 500 million of them being obese in the year 2008(179). The disease comes along with chronic inflammation, type 2 diabetes, CVD and certain types of cancer(Reference Fleissner, Huebel and Abd El-Bary180). According to the WHO(179), 65 % of the world's population live in countries where being overweight or obese accounts for more deaths than underweight. Since humans are adapted to a situation of insufficiency of energy-dense foods, and the body is better prepared to protect against weight loss than weight gain, the oversupply of such foods is the main factor causing obesity(Reference Marik9). Genetic factors may play a role as well, but an unhealthy lifestyle including so-called modern ways of nutrition such as fast food consumption, usually low in dietary fibre but high in fat and sugar, together with a lack of physical activity undoubtedly contributes to its development. Furthermore, dietary intake also affects gut ecology and bacterial composition, potentially contributing to the development of the disease as well(Reference Greiner and Bäckhed181). According to recent studies carried out with rodents and human subjects, differences in the gut microbiome of obese and non-obese subjects, and diet-dependent changes in the bacterial composition could be shown(Reference Ley, Backhed and Turnbaugh3, Reference Duncan, Lobley and Holtrop7). Apparently, differences in microbial composition are correlated with changes in its metabolic function, as in obese rodents increased levels of fermentation endproducts and lower energy contents were detected in the faeces, in comparison with non-obese animals(Reference Turnbaugh, Ley and Mahowald1). An obesity-associated microbiota seems to increase fermentation of polysaccharides, which actually results in an enhanced energy yield for the host(Reference Turnbaugh, Ley and Mahowald1). In human studies, butyrate levels in stool samples were lower in lean participants(Reference Schwiertz, Taras and Schafer182), and the number of butyrate-producing bacteria (Firmicutes phyla) decreased following the consumption of a diet designed to induce weight loss(Reference Duncan, Lobley and Holtrop7, Reference Nadal, Santacruz and Marcos183). Differences in the gut microbiota composition between lean and obese individuals have been studied in rodents and human subjects. In mice, higher numbers of the bacterial phylum Firmicutes were present in obese subjects in comparison with normal or lean animals. Of Bacteroidetes, lower numbers were generally observed in obese mice(Reference Turnbaugh, Ley and Mahowald1, Reference Ley, Backhed and Turnbaugh3), which corresponds to findings in human subjects(Reference Turnbaugh, Hamady and Yatsunenko184). Moreover, lower quantities of the Bifidobacterium group were found in overweight humans(Reference Schwiertz, Taras and Schafer182), and there is evidence that reduced colonisation of the GIT with bifidobacteria in early childhood is correlated with obesity(Reference Kalliomäki, Collado and Salminen185).
However, inconsistencies between the results of these studies have to be acknowledged, as summarised in Table 5(Reference Turnbaugh, Ley and Mahowald1, Reference Duncan, Lobley and Holtrop7, Reference Schwiertz, Taras and Schafer182). For example, Bacteroidetes numbers were found to be lower in obese compared with lean mice(Reference Turnbaugh, Ley and Mahowald1), while they occurred in higher numbers in obese compared with lean human subjects in the study of Schwiertz et al. (Reference Schwiertz, Taras and Schafer182), but were less abundant in obese humans according to Turnbaugh et al. (Reference Turnbaugh, Hamady and Yatsunenko184). Possible reasons for these discrepancies might result from the use of rodent models on the one hand due to limitations inherent to apparent metabolic and physiological differences between rodents and humans, or due to differences concerning adipose tissue biology(Reference Houpt, Houpt and Pond11, Reference Arner186). On the other hand, when carrying out obesity studies with human subjects, it has to be considered that the use of human subjects is limited and the standardisation of the experimental conditions may be rather restricted. When using the pig as an animal model, possible obesity–microbiota interactions can be assessed under more controlled conditions of feed intake compared with investigations using human subjects, thereby maintaining standardised experimental conditions. In addition to similarities of digestive function and nutritional requirements, the major contributor to fat mass is the subcutaneous adipose tissue in both pigs and humans(Reference Houpt, Houpt and Pond11). In addition, fat cell size and body fat distribution are similar in both species(Reference Houpt, Houpt and Pond11) and the pig's propensity to sedentary behaviour and fattening is comparable with that of humans(Reference Torres-Rovira, Astiz and Caro187). Nevertheless, it has to be considered that the major site of lipogenesis in the pig is the adipose tissue(Reference O'Hea and Leveille188), while in humans it occurs mainly in the liver(Reference Letexier, Pinteur and Large189), and the rapid growth and mature size of modern swine breeds to obtain maximal performance have to be taken into account when comparing the two species(Reference Rispat, Slaoui and Weber32). Still, the present, rather lean pig breeds will respond to a low-protein or high-fat diet by depositing more fat(Reference Mitchell190).
Table 5 Bacteria phyla in obese mice, humans and pigs compared with normal-weight individuals

RRNA, ribosomal RNA; ↓ , lower; ↑ , higher; qPCR, quantitative PCR; ↔ , no difference.
* Genetically obese animals.
In this context, Guo et al. (Reference Guo, Xia and Tang2) conducted a study with a Banna mini-pig inbred line, which shows pathological phenotypes of obesity and thinness. Lower numbers of Bacteriodetes in faecal samples were associated with normal rather than with obese pigs (Table 5)(Reference Guo, Xia and Tang2). These findings are in agreement with the results of a study with human participants, where obese subjects showed fewer numbers of Bacteroidetes and more Firmicutes compared with lean ones(Reference Ley, Turnbaugh and Klein191). In this study, an increased abundance of Bacteroidetes was correlated with loss of BW, both following consumption of a carbohydrate- or fat-restricted diet(Reference Ley, Turnbaugh and Klein191). In a recent study with Ossabaw mini-pigs, the obese group, which was fed a high-energy diet, also showed a higher abundance of Firmicutes in the terminal ileum, and lower abundance of Bacteroidetes in the colon than lean Ossabaw mini-pigs(Reference Pedersen, Ingerslev and Sturek192). In addition, obese Ossabaw mini-pigs had lower abundances of the genera Prevotella and Lactobacillus and a higher abundance of Clostridium spp. in colon digesta than the lean animals. However, when in the same study Göttingen mini-pigs rather than Ossabaw mini-pigs were used, opposite results were obtained concerning the ratio of Firmicutes to Bacteroidetes, with a higher abundance of Firmicutes in the lean group. According to the authors of the study, certain bacterial groups such as Firmicutes may flourish best under high-fat-diet conditions as observed in Ossabaw mini-pigs, while others like Bacteroides prosper under diet conditions such as overeating as observed in Göttingen mini-pigs(Reference Pedersen, Ingerslev and Sturek192). Additionally to research on these main bacterial groups, the prevalence of faecal methanogens of lean-breed Landrace pigs in comparison with obese-breed Erhualian pigs was determined(Reference Luo, Su and Wright193). These authors found a higher density and diversity of methanogens in the faecal samples of the lean breed. Since the formation of methane has been associated with energy loss in ruminants(Reference Johnson and Johnson194), a highly dense and diverse methanogen community may also indicate energy loss in single-stomached animals, thereby affecting energy metabolism and body fat mass formation(Reference Luo, Su and Wright193). Similarly, anorexic patients exhibited an even higher diversity of Methanobrevibacter smithii compared with lean subjects(Reference Armougom, Henry and Vialettes195). Moreover, He et al. (Reference He, Ren and Kong196) used genetically obese pigs (Ningxiang strain) as an animal model for childhood obesity, and compared them with lean (Duroc × Landrace × Large Yorkshire strain) growing pigs. Among other serum metabolites, they found reduced concentrations of trimethylamine-N-oxide, and increased concentrations of choline in the serum of obese compared with lean pigs. These metabolites have eventually been associated with functions of the gut microbiota(Reference Rezzi, Ramadan and Fay197, Reference Li, Wang and Zhang198), thus indicating a possible relationship between the development of obesity and modulated microbial nutrient metabolism. Earlier, Varel et al. (Reference Varel, Pond and Pekas199) used genetically obese and lean pigs to study the effect of low or high dietary fibre content on cellulolytic bacteria numbers. According to the authors, the obese pigs showed a tendency for lower numbers of cellulolytic bacteria in faecal samples following consumption of the high-fibre diet compared with the lean pigs, probably due to the faster digesta passage rate observed in obese pigs. In conclusion, these studies confirm the potential of pigs to serve as a model animal for obesity research.
Dietary strategies as therapeutic tools for obesity
To possibly overcome obesity, different pre- and probiotics have been assessed for their potential to modulate gut bacteria associated with obesity(Reference Abrams, Griffin and Hawthorne200, Reference Kadooka, Sato and Imaizumi201); however, until now mainly rodent models or human subjects have been used. Prebiotic components with a positive impact on obesity include oligofructose, which stimulated caecal bifidobacteria numbers in mice fed a high-fat diet and reduced the metabolic disease(Reference Cani, Neyrinck and Fava202). In a study with rats, An et al. (Reference An, Park and Lee203) found beneficial anti-obesity effects due to supplementation with the probiotic Bifidobacterium spp. to a high-fat diet (Bifidobacterium pseudocatenulatum SPM 1204, Bifidobacterium longum SPM 1205, Bifidobacterium longum 1207), with reduced body and fat weights and blood serum levels (for example, TAG, glucose, leptin) and increased total faecal lactic acid bacteria counts in the probiotic-supplemented high-fat diet compared with the diet devoid of probiotics.
In young adults, dietary supplementation with oligofructose in combination with inulin caused a smaller increase in BMI and total fat mass(Reference Abrams, Griffin and Hawthorne200). As a probiotic strain, L. rhamnosus applied as a perinatal intervention diminished the initial phase of excessive weight gain in infants(Reference Luoto, Kalliomaki and Laitinen204), while L. gasseri in fermented milk significantly decreased visceral and subcutaneous fat areas as well as BW of overweight human participants(Reference Kadooka, Sato and Imaizumi201). Potential effects of these food additives on the gut microbiota composition were not determined in the aforementioned studies.
In addition to the application of food additives such as pro- and prebiotics, modulating diet composition has been shown to be effective as well, since a decrease in Firmicutes in faecal samples was observed for a high-protein/low-carbohydrate diet conceived for weight loss in human subjects(Reference Duncan, Lobley and Holtrop7). Conversely, an increase of Firmicutes was induced by a high-fat/high-sugar Western diet in mice(Reference Turnbaugh, Baeckhed and Fulton205). The use of a porcine model may have advantages due to the limitations of rodents and human subjects in disease research, because of the metabolic and physiological differences between humans and rodents(Reference Houpt, Houpt and Pond11, Reference Arner186), and limitations of availability and standardisation of experimental conditions for human models. This is underlined by the inconsistencies observed amongst studies using rodents and human subjects, emphasising the need for a more reliable and standardised animal model, as could be provided by the pig. Thus, studies concerning food additives to be applied in humans have been tested in the pig as well. Accordingly, Wall et al. (Reference Wall, Ross and Shanahan206) investigated the impact of orally administered Bifidobacterium breve in combination with linoleic acid in sunflower-seed oil on the fatty acid composition both of murine and porcine liver and adipose tissues. Here, analysis of pigs' faeces, and small- and large-intestinal contents confirmed the GI transit and survival of the administered Bifidobacterium strain. Furthermore, in porcine livers, following the supplementation of Bifidobacterium breve and linoleic acid, a 1·5-fold higher cis-9, trans-11-conjugated linoleic acid (CLA) content was found compared with unsupplemented control animals(Reference Wall, Ross and Shanahan206). The addition of Bifidobacterium breve also led to an increase of cis-9, trans-11-CLA in the adipose tissue of these pigs, but the increase was not significantly different from that in the controls. These results are of therapeutic relevance, since CLA has been shown to improve non-alcoholic fatty liver disease, a condition accompanying obesity, in rats and human patients(Reference Wall, Ross and Shanahan206–Reference Loguercio, Federico and Tuccillo208). Thus, elevation of cis-9, trans-11-CLA in the liver as produced by Bifidobacterium breve in this trial might be a possibility for treating liver dysfunctions of that kind in humans(Reference Wall, Ross and Shanahan206). In another study, Andersen et al. (Reference Andersen, Mølbak and Thymann21) investigated the effects of dietary long-chain n-3 PUFA supplementation from fish oil to the diet of piglets. In contrast to similar studies with rodents(Reference Baillie, Takada and Nakamura209, Reference Huber, Loffler and Bilban210) and human subjects(Reference Kabir, Skurnik and Naour211, Reference Hill, Buckley and Murphy212), these authors, as well as Kratz et al. (Reference Kratz, Callahan and Yang213) in a study with human subjects, failed to obtain any effect on adipose tissue mass in the pig model(Reference Andersen, Mølbak and Thymann21). Also, a correlation between the caecal content of Bacteroides spp. and fat mass as monitored before in human subjects, mice and pigs(Reference Guo, Xia and Tang2, Reference Ley, Backhed and Turnbaugh3, Reference Ley, Turnbaugh and Klein191) could not be determined(Reference Andersen, Mølbak and Thymann21). However, the authors observed an influence of dietary PUFA on the overall bacterial community in the caecum, as shown for the faecal bacteria of human infants as well(Reference Nielsen, Nielsen and Lauritzen214), and a degrading effect of n-3 PUFA on the Bacteroidetes community(Reference Andersen, Mølbak and Thymann21). Generally, the pig model seems to be promising in research concerning the assessment of dietary strategies to improve the obesity syndrome, due to the described advantages compared with rodents and human models.
Further options for the application of the pig as a model for research into microbiota-associated diseases
Another application area for the use of pigs as a model for microbiota-associated diseases is the pathogenesis of Helicobacter pylori infection, which is a major reason for the genesis of gastritis and peptic ulcers in humans(Reference Bayerdorffer, Oertel and Lehn215, Reference Graham216). The use of diverse animal models was reviewed by Kusters et al. (Reference Kusters, van Vliet and Kuipers217), who pointed out the advantages of the gnotobiotic piglet, as it is a single-stomached mammal with similar needs in nutrition, and has a stomach with similar anatomical and physiological characteristics compared with humans. Colonisation of gnotobiotic piglets with H. pylori results in gastritis and gastric ulcers, and with the porcine model, the importance of H. pylori urease activity and motility for colonisation, besides other virulence factors, could be proved. Furthermore, antimicrobial therapies and the application of vaccination have been tested with this model. However, despite these promising results about the use of pigs as a model for research in gastritis and gastric ulcers, nowadays, for unspecified reasons, the pig is no longer used as model for research in this field(Reference Kusters, van Vliet and Kuipers217).
Possible improvement of the pig as an animal model: human flora-associated pigs
Recently, research directed to the use of pigs as an animal model also included human flora-associated (HFA) animal models by transplantation of human gut microbiota into gnotobiotic animals, both in pigs and rodents(Reference Oozeer, Goupil-Feuillerat and Alpert218–Reference Pang, Hua and Yang220). However, in rodents, due to the apparent differences in anatomy and physiology compared with humans, some key members of the human gut microbiota such as bifidobacteria do not colonise the rodent gut. Therefore, according to Pang et al. (Reference Pang, Hua and Yang220), results based on the use of rodent models often appear to be hardly relevant for humans. On the contrary, Pang et al. (Reference Pang, Hua and Yang220) successfully used the pig as a HFA animal model. Here, it has been observed that DNA fingerprints of HFA piglets were more similar to those of humans than to those of conventionally raised piglets. Moreover, with Bacteroides spp. and bifidobacteria, two important bacterial groups of the human gut were effectively established in the GIT of the pigs(Reference Kabir, Skurnik and Naour211). A significant increase in the amount of bifidobacteria spp. in HFA compared with pig flora-associated pigs has also been observed by Che et al. (Reference Che, Pang and Hua221). Accordingly, Shen et al. (Reference Shen, Zhang and Wei222) described the HFA piglet as ‘a significantly improved model for research on human gut microbiota’. In their study, the modulating effects of prebiotic FOS on faecal microbiota have been assessed, as by confirming the bifidogenic properties of short-chain FOS(Reference Shen, Zhang and Wei222). For that reason, HFA pigs might have the potential to mimic the human gut microbiota even more authentically, and thus may be used more frequently as an animal model in the future.
Conclusions
The pig has already been used in many studies as an animal model for humans to assess the gut microbiota, due to similarities in GIT functions and anatomical structure, metabolism and nutritional requirements, but also due to similar major bacteria phyla occurring in the GIT of pigs (Firmicutes, Bacteroidetes). However, considerable differences in bacterial composition have to be accounted for, which may at least in part be attributed to differences between pigs and humans, for example, relating to environmental aspects or characteristic behaviour.
With regard to research on dietary modulation of the intestinal microbiota as a therapeutic or preventive tool, a recent study confirmed the similarity of the pig to humans in view of genetic and protein malfunctions as accounting for obesity and other distinct symptoms such as diabetes(Reference Groenen, Archibald and Uenishi223). Furthermore, the pig might serve as a suitable animal model for studying other diseases such as colon cancer. For example, dietary fibre may exhibit a protective effect against the development of colon cancer(224), since its consumption appears to be associated with a diluting effect on carcinogens due to an increased faecal bulk(Reference Weisburger, Reddy and Rose225), as well as with the stimulation of butyrate production, an effective promoter of epithelial growth in the large intestine(Reference Velazquez, Seto and Bain226). As dietary stimulation of intestinal butyrate production has also been shown in pigs(Reference Le Gall, Serena and Jørgensen227), it can be suggested that the pig as an model animal could be a useful option when investigating dietary strategies to beneficially affect microbial ecology for human health purposes. However, research is still needed to further scrutinise the efficient use of pigs in research directed to serve human needs. Within this regard, the use of HFA pigs should be considered to possibly improve the pig as a model for humans.
Acknowledgements
We thank Silke Hoerner for assisting in preparing the manuscript.
The present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. S. N. H. is supported by a doctoral scholarship awarded by the Faculty of Agricultural Sciences of the University of Hohenheim. The Faculty of Agricultural Sciences of the University of Hohenheim had no role in the design, analysis or writing of this article.
R. M. and E. W. are responsible for the conceptualisation and implementation of the manuscript. S. N. H. wrote the manuscript. All authors reviewed the manuscript and approved submission.
There are no conflicts of interest.