Introduction
Species with specific roosting, foraging and/or breeding requirements tend to be particularly vulnerable to habitat loss and degradation (e.g. Jones et al., Reference Jones, Purvis and Gittleman2003; Cardillo et al., Reference Cardillo, Mace, Jones, Bielby, Bininda-Emonds and Sechrest2005; Davidson et al., Reference Davidson, Hamilton, Boyer, Brown and Ceballos2009). In the case of bats, the availability and environmental condition of caves can be a limiting factor for several species (Glover & Altringham, Reference Glover and Altringham2008), and the conservation of caves is frequently highlighted as being a priority for bats (Arita, Reference Arita1996; Goodman et al., Reference Goodman, Andriafidison, Andrianaivoarivelo, Cardiff, Ifticene and Jenkins2005; Kingston, Reference Kingston2010; Bernard et al., Reference Bernard, Aguiar, Brito, Cruz-Neto, Gregorin, Machado, Freitas and Vieira2012).
Endemic to the Neotropics, species of the family Natalidae are considered to be cave specialists (Tejedor, Reference Tejedor2011). These insectivorous bats have funnel-shaped ears, and their tails are usually longer than their total body length (Gardner, Reference Gardner and Gardner2008; Tejedor, Reference Tejedor2011). All males have a natalid organ, located on the dorsal surface of the muzzle, which secretes a substance that can facilitate intraspecific communication (Tejedor, Reference Tejedor2011). Bats of this family are light (2–12 g) and small (forearm length 26–51 mm; Tejedor, Reference Tejedor2011). Currently, there are three extant recognized genera in the family: Chilonatalus, Natalus and Nyctiellus (Morgan & Czaplewski, Reference Morgan and Czaplewski2003; Tejedor, Reference Tejedor2011; Nogueira et al., Reference Nogueira, Lima, Moratelli, Tavares, Gregorin and Peracchi2014).
Eight species of Natalus are known: N. jamaicensis, N. lanatus, N. macrourus, N. major, N. mexicanus, N. primus, N. stramineus and N. tumidirostris (Tejedor, Reference Tejedor2011). In South America, south of the Amazon River, only the species N. macrourus (formerly known as N. espiritosantensis) is known to occur, in Bolivia, Brazil and Paraguay (Tejedor, Reference Tejedor2011; Garbino & Tejedor, Reference Garbino and Tejedor2012). Natalus macrourus is categorized as Near Threatened on the IUCN Red List (Tejedor & Davalos, Reference Tejedor and Davalos2016) but it is one of the six bat species officially designated as threatened in Brazil, where it is categorized as Vulnerable on the basis of a projected population reduction associated with a decline in its area of occupancy, extent of occurrence and/or quality of habitat (criterion A3c; MMA, 2014). Although N. macrourus has a larger distribution than other congeneric species, records are rare within its range (Tejedor, Reference Tejedor2011), and there is still a paucity of knowledge about its biology, ecology and natural history.
In this study we (1) addressed gaps in the known distribution of N. macrourus in Brazil, obtaining new records for the north-eastern region, (2) updated data on the distribution of the species in Brazil, (3) used these data together with climate and environmental modelling to generate a refined map of the potential distribution of the species in Brazil, (4) analysed pressures on and threats to the species, and (5) assessed its conservation needs.
Methods
Current and potential distribution
We reviewed the available records of N. macrourus in Brazil by searching the scientific literature for the key words Natalus and Natalidae in Web of Science (2015) and Google Scholar (2015). In addition, we searched for records in the databases of the Chico Mendes Institute for Biodiversity Conservation (ICMBio, 2016), speciesLink (2015), VertNet (2015) and the Global Biodiversity Information Facility (GBIF, 2015; Supplementary Table S1).
The records obtained from the literature were converted into points of occurrence and used to model the potential distribution of N. macrourus in Brazil (Supplementary Table S1). Each record was checked and filtered for mistakes in location and/or taxonomy (Peterson et al., Reference Peterson, Soberón, Pearson, Anderson, Martínez-Meyer, Nakamura and Araújo2011). Points that are too close and under the same environmental conditions can bias the modelling as a result of so-called spatial autocorrelation (Boria et al., Reference Boria, Olson, Goodman and Anderson2014). To reduce the inherent geographical biases associated with collection data and avoid spatial autocorrelation problems, we produced a map of environmental heterogeneity, using the bioclimatic variables available from WorldClim (2015), and removed records that were within 25 km of one another under the same environmental conditions, keeping the maximum possible number of localities. This resulted in 50 single localities.
Using MaxEnt (Phillips et al., Reference Phillips, Anderson and Schapire2006), we generated various distribution models for N. macrourus, based on two sets of variables at 1 km2 resolution. To avoid collinearity among bioclimatic variables (i.e. when two variables are highly correlated) we calculated Pearson correlations for the 22 variables available in the WorldClim database and, for those with ≥ 80% correlation, eliminated the one with the lowest contribution. Following this selection, for the first set of variables we considered nine bioclimatic variables derived from temperature and rainfall (mean daily temperature range, isothermality, temperature seasonality, maximum temperature in the warmest month, mean temperature in the wettest quarter, mean temperature in the coldest quarter, annual rainfall, rainfall in the driest quarter, rainfall in the warmest quarter). In the second set we considered the same nine bioclimatic variables plus altitude, the normalized difference vegetation index (a proxy for measuring vegetation cover) and a categorical variable of the potential occurrence of caves in Brazil, produced by the National Centre for Cave Research. The shapefile for the potential occurrence of caves in Brazil was produced using data on the location of the main karst regions of Brazil, the geological map of the country, georeferenced records of caves in the database of the National Centre for Cave Research, and the main lithological formations of the caves. We used this approach because N. macrourus is generally regarded as a cave-dwelling species (Tejedor & Davalos, Reference Tejedor and Davalos2016). Considering that only a small fraction of Brazilian caves are known, we adopted potential occurrence as a proxy for cave existence in a given area. The National Centre for Cave Research categorizes the potential of cave occurrence in Brazil as unlikely, low, medium, high and very high. In our analysis we scored these categories 0–4, and used the scores to refine the modelling of the potential distribution of N. macrourus.
We conducted various tests to find the best parametrization for MaxEnt (Radosavljevic & Anderson, Reference Radosavljevic and Anderson2014). We set the program to use 80% of the data to calibrate the model and 20% for the test, using n − 1 replicates, where n is the number of records of occurrence, as suggested by Pearson et al. (Reference Pearson, Raxworthy, Nakamura and Peterson2007). To assess the predictive capacity of the models we used the area under the curve (AUC); the best-performing models had AUC values close to 1, whereas AUC values close to 0.5 indicated models were equal to or worse than random (Phillips & Dudík, Reference Phillips and Dudík2008).
Conservation scenarios for the species
To mitigate the occurrence of false positives (commission errors), the potential distribution generated included a buffer of 300 km around the minimum convex polygon produced with known points of occurrence. Considering the minimum training presence threshold (i.e. the lowest predicted value associated with any one of the observed presence records; Peterson et al., Reference Peterson, Soberón, Pearson, Anderson, Martínez-Meyer, Nakamura and Araújo2011; Radosavljevic & Anderson, Reference Radosavljevic and Anderson2014), our analysis indicated 18% as the threshold for the presence of Natalus. Therefore, we categorized the potential distribution according to occurrence probability: very low (< 18%), low (18–25%), medium (26–50%), high (51–75%) and very high (> 75%). We overlapped the potential distribution generated with three other datasets: (1) deforested areas in Brazil until 2009 (SISCOM, 2015), (2) potential occurrence of caves (CECAV, 2015), and (3) boundaries of fully protected areas as of 2011 (MMA, 2016). This overlap facilitated the calculation of the area of occupation of the species, the number of potential roosts within that area, and the occurrence distribution within protected areas. We also calculated the percentage of caves within the range of the species in habitat remnants for the entire country and for the north-eastern region, and the percentage of caves potentially under pressure from mining activities (DNPM, 2015) and wind farms (ANEEL, 2015).
Results
Known and new records
Our literature survey yielded 81 records of N. macrourus in Brazil (Fig. 1; Supplementary Table S1). We added new records for the north-eastern state of Pernambuco, obtained from captures made during a bat inventory in the cave Meu Rei in Catimbau National Park (62,292 ha), which is located within the Caatinga biome (ICMBio, 2015). The cave is within a sandstone formation, with a horizontal length of 162 m (Azevedo & Bernard, Reference Azevedo and Bernard2015). It harbours a bat community that includes at least seven other species of two families (Phyllostomidae: Diphylla ecaudata, Carollia perspicillata, Glossophaga soricina, Anoura geoffroyi, Lonchorhina aurita, Tonatia bidens; and Mormoopidae: Pteronotus gymnonotus), which at certain times can surpass 120,000 individuals, most of them P. gymnonotus. Measurements in two chambers of the cave, one in its central part and the other in a deeper part, yielded mean temperatures of 25 and 28°C, and relative humidity of 80 and 87%, respectively. The cave is a high priority for full protection (Azevedo & Bernard, Reference Azevedo and Bernard2015).
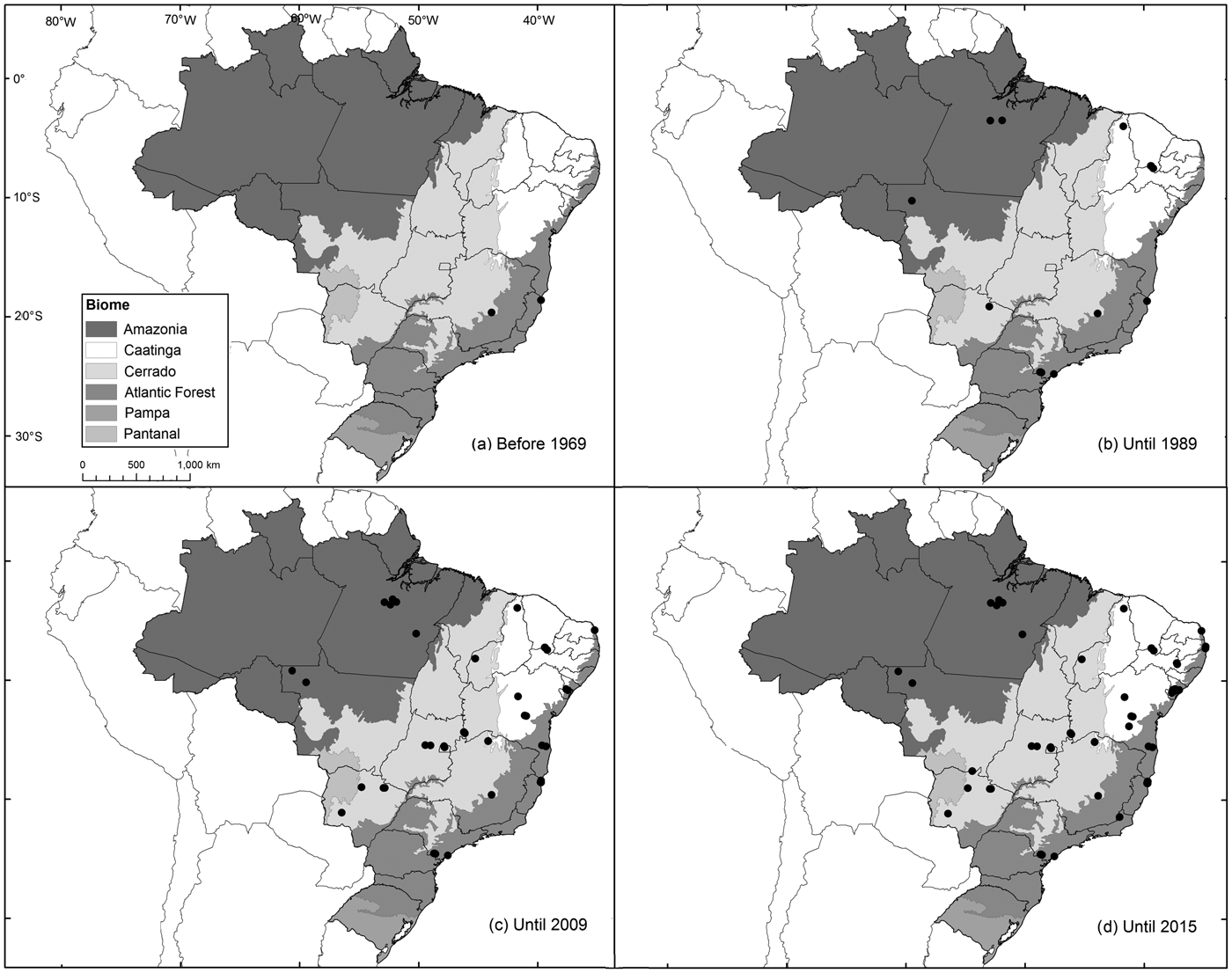
Fig. 1 Spatial evolution of records of the Brazilian funnel-eared bat Natalus macrourus (Natalidae) from 1893 to 2015.
Using hand nets we captured eight N. macrourus (4 males and 4 females) in the cave Meu Rei. The captures were made in October and December 2014, and March, May, June, August and October 2015. Three of these individuals were collected; the others were not marked, so recaptures were possible. We weighed and measured each bat and estimated its age and reproductive status, but retained only the first individual as a voucher, which we deposited in the Mammal Collection of the Federal University of Pernambuco (UFPE 3317; ICMBio/MMA permit #43816-1; Ethics Committee on Animal Care—UFPE #23076.027916/2015-13). All the other bats were released at the site of capture. We recorded another individual of N. macrourus on 14 May 2015 during a visit to another cave 11 km away, outside the limits of Catimbau National Park.
The records from Pernambuco were added to the existing records of the species in Brazil for 1893–2015 (Fig. 2). Sixty percent of these records are from caves, grottos or within 5 km of known caves, and 70% are from within 10 km of known caves. Thirty percent of all records are from ecotones between savannah, steppic savanna, dry coastal vegetation (restinga), or croplands, 25% are from dense rainforests, and 20% are from savannahs. The records are broadly distributed and occupy four of the six Brazilian biomes (Fig. 1). Most records are from Atlantic Forest (40%), followed by Cerrado (23%), Amazonia (17%) and Caatinga (16%). In 2015 N. macrourus was included in the species list for the Pantanal, based on records in the state of Mato Grosso do Sul (Fischer et al., Reference Fischer, Santos, Carvalho, Camargo, Cunha and Silveira2015).
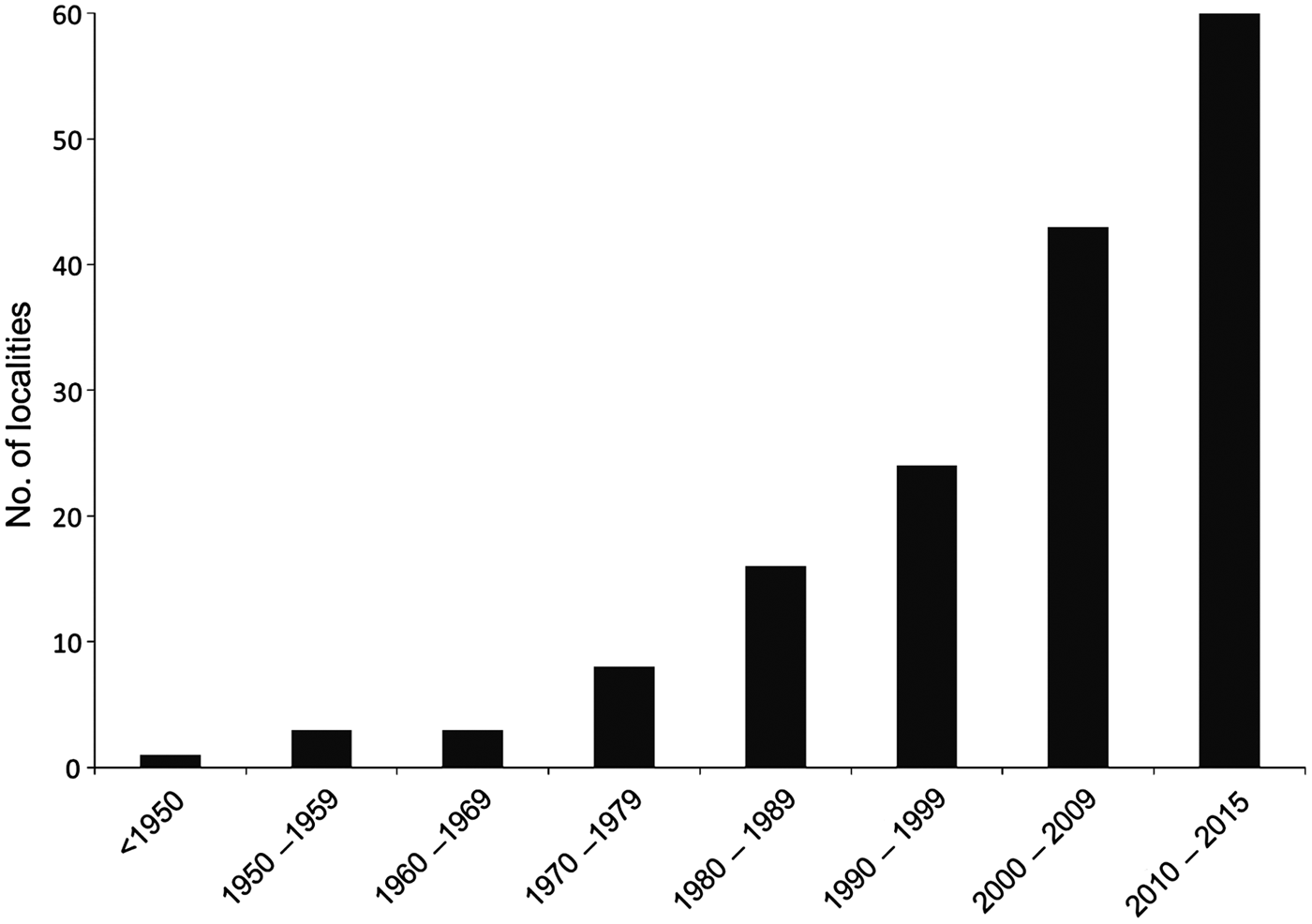
Fig. 2 Temporal evolution of the number of localities with known records of the Brazilian funnel-eared bat in Brazil from 1893 to 2015.
Potential distribution modelling
The potential distribution of N. macrourus modelled using bioclimatic variables combined with the potential occurrence of caves was 4,445,996 km2 (AUCtraining = 0.89 ± SD 0.01; AUCtest = 0.87 ± SD 0.05), which is 11.5% smaller than the potential distribution modelled using bioclimatic variables only, which was 5,024,815 km2 (AUCtraining = 0.86 ± SD 0.01; AUCtest = 0.85 ± SD 0.04). However, considering the potential distribution based on both bioclimatic variables and the potential occurrence of caves there was a reduction of 26% in the areas of highest environmental suitability (> 75%) for the species when compared with the model based on bioclimatic variables only. Our model suggests that N. macrourus is positively associated with areas where there is a high potential occurrence of caves, and negatively associated with areas with high variation in mean daily temperature and mean annual rainfall.
The areas of highest environmental suitability for N. macrourus corresponded to only 3% of the total area of potential distribution, and these areas were within the Caatinga and Atlantic Forest biomes, mostly in the north-eastern region. That region alone comprised 67% of the total area of highest environmental suitability in the model that included the potential occurrence of caves, and 92% in the model with bioclimatic variables alone (Fig. 3). In the Atlantic Forest, the areas of highest environmental suitability were located in Sergipe, Alagoas, eastern Bahia, and mid-eastern Pernambuco and Paraíba states. Additionally, the areas of high environmental suitability (50–75%) accounted for < 20% of the total area modelled, and were located mostly in the Atlantic Forest of north-eastern Brazil (55% in the model including the potential cave occurrence variable; 46% without). Areas of low environmental suitability (18–25%) were found in north-western Bahia, southern Piauí and eastern Amazonas (to the south of the Amazon River) states, as well as in mid-northern Mato Grosso, Tocantins and Paraná states.
Conservation scenarios for the species
Based on the model of potential distribution of the species, considering the importance of caves for N. macrourus and considering the area where original vegetation cover was already lost, we estimate that the species has already lost 54% of its habitat in Brazil and that there are < 35% of habitat remnants in areas with the highest environmental suitability (Fig. 3). We estimate that 53% of the caves recorded within the distribution of N. macrourus are in habitat remnants and c. 54% are < 5 km from mining operations. Furthermore, 2% of these caves are < 10 km from wind farms, and only 4% of the potential distribution of N. macrourus is located within fully protected areas.
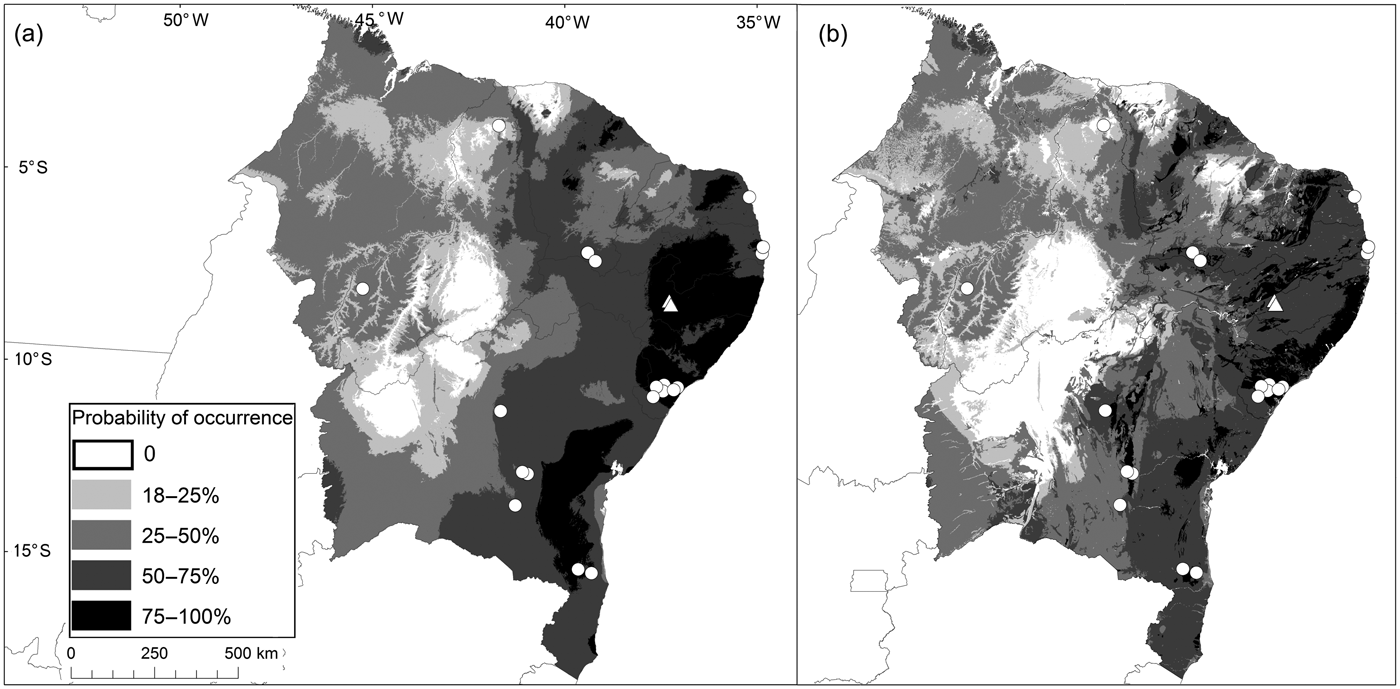
Fig. 3 Potential distribution of the funnel-eared bat N. macrourus in north-eastern Brazil, modelled considering only bioclimatic variables (a), and with the addition of cave occurrence potential (b). White circles represent known records for the species, and white triangles represent new records for the state of Pernambuco.
Approximately 30% of the area of potential distribution of N. macrourus is located in north-eastern Brazil, where 44% of the caves are in human-modified areas. We estimate that N. macrourus has already lost 50% of its natural habitat in north-eastern Brazil, and up to 65% in areas of highest environmental suitability in the eastern part of the region, where human population growth is higher. In that region only 2% of the potential distribution of N. macrourus is located within fully protected areas.
Discussion
The records of N. macrourus indicate a broad distribution in Brazil, with the species occurring from xeric (e.g. the Caatinga, with annual rainfall < 800 mm) to moist habitats (e.g. the Amazon, with annual rainfall > 2,000 mm; Tejedor, Reference Tejedor2011). However, although our model suggests a large potential distribution, most of the records in Brazil are from areas of open vegetation, and our models suggest a preference for areas with low mean annual rainfall, lower variation in daily temperature, and high cave occurrence potential. These preferences may significantly restrict the effective area of occurrence of N. macrourus. We observed that only 3% of the total area of potential distribution has high environmental suitability for the species, and is located in the Caatinga and Atlantic Forest, mostly in north-eastern Brazil.
Brazil may have > 310,000 caves (Piló & Auler, Reference Piló and Auler2011) but < 5% of them have been officially recorded (ICMBio/CECAV, 2016). Species of the genus Natalus are considered to be dependent on caves for roosting (Taddei & Uieda, Reference Taddei and Uieda2001; Tejedor et al., Reference Tejedor, Silva-Taboada and Rodríguez-Hernández2004; Tejedor & Davalos, Reference Tejedor and Davalos2016), and our analysis confirms this strong association. This finding is a cause for concern, as changes in the Brazilian cave protection law have increased the vulnerability of cave environments and may result in a poor outlook for the conservation of cave-dependent species. Until 2008, Brazilian caves were fully protected. However, the law was changed by Presidential Decree no. 6,640, which determined that caves should be categorized according to their relevance, and only those categorized as being of ‘maximum relevance’ would be fully protected (Brasil, 2008). The prior categorization of all the Brazilian caves as a prerogative for their protection is infeasible and, in practice, the change in the law has reduced their protection, as caves in the categories of high, medium and low relevance may be legally exploited and destroyed. Decree no. 6,640 is therefore considered to be a serious threat to the conservation of Brazilian bats (Bernard et al., Reference Bernard, Aguiar, Brito, Cruz-Neto, Gregorin, Machado, Freitas and Vieira2012). Cave protection is highlighted as being a priority for bat conservation (e.g. Fenton, Reference Fenton1997; Luo et al., Reference Luo, Jiang, Lu, Wang, Wang and Feng2013; Furey & Racey, Reference Furey, Racey, Voigt and Kingston2016) and an increase in the number of formally protected roosts is needed urgently in Brazil, given the pressure on cave environments there (e.g. Ribeiro, Reference Ribeiro2015).
As well as depending on caves for shelter, Natalus bats, which are strictly insectivorous, also depend on having tracts of habitat of sufficient environmental quality available for foraging. Therefore, N. macrourus may face other pressures in addition to roost loss. Our results suggest that c. 54% of the known caves within the potential distribution of N. macrourus are < 5 km from mining areas. Besides the direct loss of shelters caused by the exploration of caves for mining, explosives frequently used in that process, the presence of people, and the noise caused by machinery can affect bats using these shelters (Furey & Racey, Reference Furey, Racey, Voigt and Kingston2016). Furthermore, 2% of these caves are < 10 km from wind farms, which are another threat to bats (Barclay et al., Reference Barclay, Baerwald and Gruver2007; Arnett et al., Reference Arnett, Brown, Erickson, Fiedler, Hamilton and Henry2008). The catchment areas of wind farms in the Neotropics are largely unknown but studies in Europe and Canada have found that wind turbines influence not only bat populations in close proximity but also those at distances of several hundreds of kilometers or even > 1,000 km (Voigt et al., Reference Voigt, Popa-Lisseanu, Niermann and Kramer-Schadt2012; Baerwald et al., Reference Baerwald, Patterson and Barclay2014). We recommend that caves with confirmed occurrence of N. macrourus close to mines or wind farms should be protected and monitored in the medium and long term.
The conservation outlook for N. macrourus would be more positive if there were a larger number of protected areas within its distribution. However, only 4% of the potential distribution of N. macrourus is located within fully protected areas. In the north-eastern region, which has the greatest potential occurrence of N. macrourus, the percentage is even lower (2%). Hence, a scenario in which low roost protection is combined with other threats, such as mining and habitat loss, degradation and fragmentation, could extitrpate some populations locally (Tejedor, Reference Tejedor2011).
Natalus spp. are frequently associated with several other bat species (Ruschi, Reference Ruschi1951; Trajano & Moreira, Reference Trajano and Moreira1991; Trajano & Gimenez, Reference Trajano and Gimenez1998; Gregorin & Mendes, Reference Gregorin and Mendes1999; Taddei & Uieda, Reference Taddei and Uieda2001). We observed N. macrourus roosting with A. geoffroyi, C. perspicillata, D. ecaudata, G. soricina, L. aurita, P. gymnonotus and T. bidens; L. aurita is also categorized as Vulnerable in Brazil. Hence, protecting the caves used by N. macrourus could also conserve other species of bats as well as the rich, highly specialized and frequently endemic cave biota (Furey & Racey, Reference Furey, Racey, Voigt and Kingston2016).
Robust databases of species records are necessary for improving potential distribution models in large and under-sampled countries, such as Brazil (e.g. Oliveira et al., Reference Oliveira, Paglia, Brescovit, de Carvalho, Silva and Rezende2016), which would, in turn, highlight priority areas for inventories and conservation (Costa et al., Reference Costa, Nogueira, Machado and Colli2010; Moreira et al., Reference Moreira, Leite, Siqueira, Coutinho, Zanon and Mendes2014; Ingberman et al., Reference Ingberman, Fusco-Costa and Monteiro-Filho2016). However, species distribution models are influenced by many factors, such as spatial resolution, environmental variables and the quality of distribution records. The addition of new records can produce distinct modelling outputs, and models based on partial datasets for species occurrence can lead conservationists or decision makers to incorrect conclusions (Aguiar et al., Reference Aguiar, Bernard, Ribeiro, Machado and Jones2016).
There are no formal records of bats for almost 60% of Brazil (Bernard et al., Reference Bernard, Aguiar and Machado2011) and there is limited knowledge of the distribution of several bat species in the country. New records, such as those reported here from Pernambuco, help to reduce these gaps for poorly known, hard to capture species, such as Natalus spp. The previous records closest to Pernambuco were from Ceará, Sergipe and Paraíba states, >235 km from our study area (Taddei & Uieda, Reference Taddei and Uieda2001; Rocha et al., Reference Rocha, Mikalauskas, Bocchiglieri, Feijó and Ferrari2013). Moreover, nearly half of the 81 records of occurrence of N. macrourus in Brazil have been gathered since 2000 (Fig. 1; Leal et al., Reference Leal, Ramalho, Miller, Filho, Neto and Nova2012), indicating that recent efforts have resulted in a significant refinement of the species’ distribution. Considering the high potential occurrence of caves in north-eastern Brazil (Jansen et al., Reference Jansen, Cavalcanti and Lamblém2012), and considering that at least three (N. macrourus, L. aurita and Furipterus horrens) of the six threatened bat species in Brazil are frequent or exclusive cave users, caves in north-eastern Brazil are a priority for bat inventories so that roosts used by Natalus or any other threatened bat species can be identified and proposed for full protection.
Acknowledgements
We thank Ana Claudia Jardelino, Evelyn Figueiredo, Frederico Hintze, Jaire Torres and Ítalo Azevedo for their help with field work, and Project Ecológico de Longa Duração Sítio Parque Nacional do Catimbau for logistical support. We thank Valéria Tavares for sharing information on records of N. macrourus. Coordenação de Aperfeicoamento de Pessoal de Nível Superior granted scholarships to M. Delgado-Jaramillo and E. Barbier, and Conselho Nacional de Desenvolvimento Científico e Tecnológico granted a fellowship to E. Bernard. The study was funded by Fundação Grupo Boticário de Proteção à Natureza (process #0983-20132). We thank Departamento de Zoologia, Centro de Ciências Biológicas da Universidade Federal de Pernambuco for supporting our studies on bats in Brazil. We thank two anonymous reviewers for their constructive comments.
Author contributions
M. Delgado-Jaramillo collected data and conducted the analysis. E. Barbier collected data. E. Bernard conceived the research. All authors contributed to writing the article.
Biographical sketches
Mariana Delgado-Jaramillo is interested in how species distribution modelling can be applied to conservation, and her work has focused on bats. Eder Barbier’s research interests include the biology, ecology and geographical distribution of hosts and parasites. Enrico Bernard’s research is focused on the ecology and conservation of Brazilian biodiversity, with an emphasis on bats.





