No CrossRef data available.
Article contents
Pro-arrhythmogenic effects of Trypanosoma cruzi conditioned medium proteins in a model of bovine chromaffin cells
Published online by Cambridge University Press: 13 August 2021
Abstract
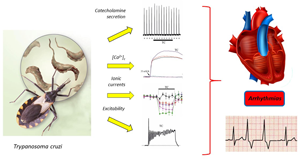
Asymptomatic sudden death is the principal cause of mortality in Chagas disease. There is little information about molecular mechanisms involved in the pathophysiology of malignant arrhythmias in Chagasic patients. Previous studies have involved Trypanosoma cruzi secretion proteins in the genesis of arrhythmias ex vivo, but the molecular mechanisms involved are still unresolved. Thus, the aim was to determine the effect of these secreted proteins on the cellular excitability throughout to test its effects on catecholamine secretion, sodium-, calcium-, and potassium-conductance and action potential (AP) firing. Conditioned medium was obtained from the co-culture of T. cruzi and Vero cells (African green monkey kidney cells) and ultra-filtered for concentrating immunogenic high molecular weight parasite proteins. Chromaffin cells were assessed with the parasite and Vero cells control medium. Parasite-secreted proteins induce catecholamine secretion in a dose-dependent manner. Additionally, T. cruzi conditioned medium induced depression of both calcium conductance and calcium and voltage-dependent potassium current. Interestingly, this fact was related to the abolishment of the hyperpolarization phase of the AP produced by the parasite medium. Taken together, these results suggest that T. cruzi proteins may be involved in the genesis of pro-arrhythmic conditions that could influence the appearance of malignant arrhythmias in Chagasic patients.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
Footnotes
A. M. Baraibar and R. de Pascual have contributed equally to this work.



