Introduction
Dipylidium caninum (Cyclophyllidea, Dipylidiidae) is a common intestinal tapeworm of dogs with a worldwide distribution and with zoonotic potential (Gates and Nolan, Reference Gates and Nolan2009; Deplazes et al., Reference Deplazes, Joachim, Mathis, Strube, Taubert, von Samson-Himmelstjerna and Zahner2021). Especially infants are prone to become infected after ingestion of infected fleas or lice (Elmonir et al., Reference Elmonir, Elaadli, Amer, El-Sharkawy, Bessat, Mahmoud, Atta and El-Tras2021) which act as intermediate hosts. In dogs, infections remain subclinical, or manifest with mild and unspecific clinical signs in most cases. Clinical signs may include anal pruritus causing an animal to rub its bottom along the ground, diarrhoea, weight loss, general restlessness or tenesmus (Wani et al., Reference Wani, Allaie, Shah, Raies, Athar and Junaid2015; Saini et al., Reference Saini, Gupta, Kasondra, Rakesh and Latchumikanthan2016). The primary drug of choice to combat infections with D. caninum in dogs is praziquantel (at a single dose of at least 5 mg kg−1 body weight (BW) per os (p.o.)) which usually shows high levels of efficacy (Schroeder et al., Reference Schroeder, Altreuther, Schimmel, Deplazes, Kok, Schnyder and Krieger2009; Saini et al., Reference Saini, Gupta, Kasondra, Rakesh and Latchumikanthan2016; ESCCAP, 2021). Another effective treatment option includes epsiprantel (at least 5.5 mg kg−1 BW p.o.) (Corwin et al., Reference Corwin, Green and Keefe1989; ESCCAP, 2021).
To date, anthelmintic resistance in canine and feline parasites has been of minor relevance and rather confined to regions and limited in scope, whereas it is a considerable problem in livestock and horses (Raza et al., Reference Raza, Rand, Qamar, Jabbar and Kopp2018; von Samson-Himmelstjerna et al., Reference Von Samson-Himmelstjerna, Thompson, Krücken, Grant, Bowman, Schnyder and Deplazes2021). Recently, an increasing frequency of reports on multiple anthelmintic resistance of Ancylostoma caninum in dog kennels in North America has been addressed as a major concern (Marsh and Lakritz, Reference Marsh and Lakritz2023). Moreover, 2 cases of possible praziquantel resistance have been reported in D. caninum-infected dogs in the United States recently (Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018; Loftus et al., Reference Loftus, Acevedo, Bowman, Liotta, Wu and Zhu2022), emphasizing the importance of this matter in companion animals as well. In Europe, no cases of anthelmintic resistance in companion animals have been reported yet. This case represents the first description of clinical resistance to praziquantel in D. caninum in Europe.
Materials and methods
Case presentation
A male, mixed breed Can de Chira dog imported from Spain of approximately 10 months of age and weighing 16.7 kg was presented with a history of chronic excretion of tapeworm proglottids after arrival in Switzerland. The dog had been probably born in December 2021 around Monzón or Huesca (municipality of Aragon, Spain) and had been picked up from the street at the approximate age of 4 months together with other dogs of the same age. Subsequently, it had been kept at an animal shelter in Monzón. Clinical signs upon presentation were mild with general restlessness, tenesmus, slight anal pruritus and occasionally squashy feces. No evidence of flea infestation was present, and the dog had received treatment against ectoparasites (fluralaner, unknown dose) and helminths (praziquantel, febantel, pyrantel, unknown dose) before entering Switzerland. In Switzerland, the dog was presented to the primary veterinarian and treated orally with 5.9 mg kg−1 BW praziquantel, 5.9 mg kg−1 BW pyrantel, 18.0 mg kg−1 BW febantel (DrontalPlus®, Vétoquinol) twice within a period of 2 weeks as well as with 14.7 mg kg−1 BW fluralaner (Bravecto®, MSD Animal Health). Flea prophylaxis with fluralaner was pursued every 12 weeks as recommended by the manufacturer. Subsequently, proglottid excretion ceased, but re-started in an unchanged manner 3 weeks after the last treatment with praziquantel/pyrantel/febantel. Hence, the dog received a single dose of 0.7 mg kg−1 BW milbemycinoxime and 7.4 mg kg−1 BW praziquantel (Milbemax®, Elanco Animal Health). Proglottid shedding continued and fenbendazole (Panacur®, MSD Animal Health) was administered orally for 5 consecutive days at a dose of 44 mg kg−1 BW. For 4 days, proglottid shedding ceased, and 0.7 mg kg−1 BW milbemycinoxime and 7.4 mg kg−1 BW praziquantel (Milbemax®, Elanco Animal Health) were administered orally twice within 2 weeks. Excretion of proglottids continued and 0.7 mg kg−1 BW milbemycinoxime and 7.4 mg kg−1 BW praziquantel (Milbemax®, Elanco Animal Health) were administered orally once a week for a period of 4 weeks, yet excretion of cestode segments persisted.
Investigations
The presence of eggs/proglottids was examined via the adhesive tape method (Deplazes et al., Reference Deplazes, Joachim, Mathis, Strube, Taubert, von Samson-Himmelstjerna and Zahner2021) and proglottids were also directly collected from the surface of the fecal samples. Furthermore, a combined sedimentation–flotation using saturated sodium chloride solution with a specific weight of 1.2 g cm−3 was performed on fecal samples (Deplazes et al., Reference Deplazes, Joachim, Mathis, Strube, Taubert, von Samson-Himmelstjerna and Zahner2021). Proglottids were assessed microscopically and egg packets were pressed out from the proglottids in squash preparations. A multiplex polymerase chain reaction (PCR) targeting the mitochondrially encoded 12S ribosomal RNA of non-Echinococcus cestodes [267 base pairs (bp)] (Trachsel et al., Reference Trachsel, Deplazes and Mathis2007) was carried out to molecularly confirm the microscopic diagnosis.
Treatments and follow-up
The patient received 31.1 mg kg−1 BW pyrantel and 12.0 mg kg−1 BW epsiprantel (Dosalid®, Zoetis) in the first place. Next, 50.3 mg kg−1 BW mebendazole (Lendue Maxi®, Teknofarma S.r.l.) were administered on 5 consecutive days. Subsequently, 6.0 mg kg−1 BW praziquantel, 6.0 mg kg−1 pyrantel and 24.0 mg kg−1 oxantel (Dolpac 10®, Vétoquinol) were initiated for a total of 6 days. One month after the last treatment with Dolpac 10®, a second round of mebendazole (Lendue Maxi®, Teknofarma S.r.l.) was administered for 5 days, this time at an increased dose of 86.2 mg kg−1. Contemporaneously, the animal owner daily recorded the development of proglottid excretion.
All the compounds mentioned in this section are not commercially available in Switzerland and were purchased in Portugal, Italy or at an international pharmacy after considering the regulations of off-label use in veterinary medicine in Switzerland and following the Swiss compendium of veterinary medicinal products (Tierarzneimittelkompendium CliniPharm, www.vetpharm.uzh.ch). The extension from 3 to 5 days of mebendazole treatment was derived from Miro et al. (Reference Miro, Mateo, Montoya, Vela and Calonge2007). A detailed temporal pattern of anthelmintic treatments and proglottid excretion is compiled in Table 1.
Table 1. Observed proglottid shedding and administered medications after antiparasitic treatments with pyrantel, febantel, milbemycinoxime, fenbendazole and fluralaner, and unsuccessful treatments with praziquantel administered to a dog infected with Dipylidium caninum. Proglottid shedding stopped for at least 10 months after the last mebendazole administration

a At his point, proglottids were not superficially located on the feces, but rather mixed within the fecal matter and they seem macerated without motility.
Results
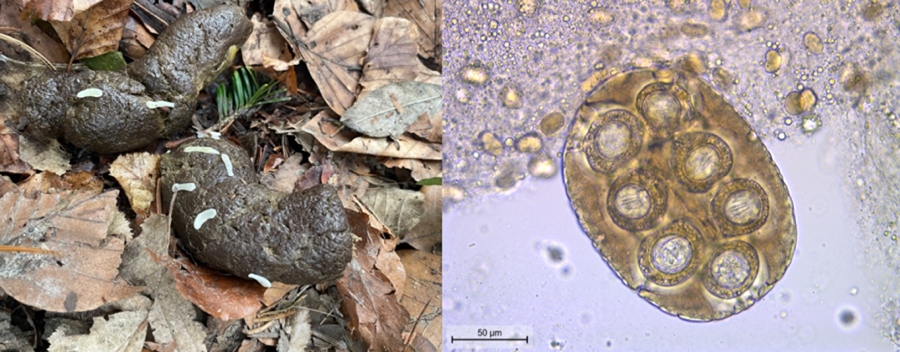
Based on gross morphological features of the proglottids (size of ~12 mm × 3 mm, mature genital organs, double genital pores slightly behind the middle of the lateral margins of each proglottid) and the eggs (capsulated and clustered in packets of 120–200 μm, with hexacanth embryo), D. caninum was diagnosed (Deplazes et al., Reference Deplazes, Joachim, Mathis, Strube, Taubert, von Samson-Himmelstjerna and Zahner2021) (Fig. 1). PCR confirmed an infection with D. caninum: a DNA segment of 259 bp was successfully sequenced and nucleotide basic local alignment search tool (BLAST) against the nucleotide collection of GenBank showed an identity of 98.07% (254/259 bp) with D. caninum from a canine host (accession number MH182479.1) (Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018). After epsiprantel/pyrantel (Dosalid®, Zoetis) treatment, proglottid shedding continued in an unchanged manner (Table 1). Mebendazole at a dose of 50.3 mg kg−1 BW for 5 consecutive days reduced the quantity of the excreted tapeworm segments. These were not superficially located on the feces anymore but mixed within the fecal matter. Moreover, the proglottids appeared macerated and without motility. Re-appearance on the surface, however, occurred 2 weeks after the final administration of mebendazole. The combination praziquantel/pyrantel/oxantel (Dolpac 10®, Vétoquinol) did not stop the excretion of tapeworm segments. Eventually, proglottid shedding stopped from the second day of treatment with mebendazole 86.2 mg kg−1 BW (Table 1) and clinical signs (general restlessness, tenesmus, slight anal pruritus, occasionally squashy feces) resolved. Moreover, the shedding of proglottids stopped and the dog remained coproscopically negative for a follow-up period of 10 months.

Figure 1. Eggs clustered in packets with hexacanth embryos typical for Dipylidium caninum.
Discussion
This case represents the first written report of apparent praziquantel resistance in D. caninum in Europe. During the investigations for potential alternative anthelmintic treatments, further oral reports from veterinary parasitologists of Italy and Spain were mentioned to the authors (M. Schnyder, personal communication 2023). To confirm that the infection was indeed caused by D. caninum, a combination of traditional parasitological and genetic methods was implemented.
Anthelmintic resistance is defined as the ability of helminth parasites to survive the administration of a certain previously effective drug (Prichard et al., Reference Prichard, Hall, Kelly, Martin and Donald1980; Sangster et al., Reference Sangster, Cowling and Woddgate2018). According to the VICH (Veterinary International Conference on Harmonization) guidelines (VICH, 2000, 2001), efficacy is described as a reduction of ⩾90% of D. caninum scolices in controlled terminal studies. In the current case resistance may be suspected by the fact that multiple doses of praziquantel at the standard label treatment of 5 mg kg−1 BW (Schmid et al., Reference Schmid, Rohdich, Zschiesche, Kok and Allan2010) did not stop the continuous excretion of proglottids. Investigating the above-mentioned threshold for a drug to be considered efficacious in a controlled study would have required experimental infections: several factors (i.e. deriving from the individual dog, or being related to the environment, etc.) may in fact influence the presence and quantity of proglottids in feces. Therefore, the absence of proglottids in this case did not allow to finally conclude on the efficacy of an anthelmintic agent, still, this represented the goal for the animal owner. Consequently, in the context of the current case, it was not possible to establish the resistance of the isolate by experimental infection of laboratory animals to further fathom the nature of this resistance, comparable to Jesudoss Chelladurai et al. (Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018). The efficacy of anthelmintic treatment in the here reported case was instead continuously assessed by documenting the presence and quantity of proglottids excreted in feces, by final determination of the absence of eggs in feces, and by the adhesive tape method. Accordingly, the isolate was not eliminated by the administration of praziquantel at the label dose of 5 mg kg−1 BW (Lloyd and Gemmell, Reference Lloyd and Gemmell1992; Altreuther et al., Reference Altreuther, Schimmel, Schroeder, Bach, Charles, Kok, Kraemer, Wolken, Yound and Krieger2009, Schroeder et al., Reference Schroeder, Altreuther, Schimmel, Deplazes, Kok, Schnyder and Krieger2009) nor by fenbendazole at a dosage of 44 mg kg−1 BW (Burke and Roberson, Reference Burke and Roberson1978) nor by epsiprantel (combined with pyrantel) at a dose of 12.0 mg kg−1 BW, i.e. more than twice as high as the recommended dose of 5.5 mg kg−1 BW (Corwin et al., Reference Corwin, Green and Keefe1989). Interestingly, oral administration of praziquantel/pyrantel/febantel twice within a period of 2 weeks induced a 3-week suspension of proglottid excretion, suggesting some efficacy. The administration of praziquantel/pyrantel/oxantel had previously been successful in D. caninum infections (Grandemange et al., Reference Grandemange, Claerebout, Genchi and Franc2007; Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018) and it was hypothesized that oxantel would exert a synergistic effect with the other compounds due to differences in drug action (Martin et al., Reference Martin, Clark, Trailovic and Robertson2004; Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018). Yet, no reduction in the excretion of proglottids was noticed. In contrast, with mebendazole at the dosage of 50.3 mg kg−1 BW for 5 days, a clear reduction of the number of proglottids on fecal samples was observed, but single proglottids were also present after the last day of treatment. Previous studies have indicated an efficacy of oral mebendazole against cestodes (Vanparijs and Thienpont, Reference Vanparijs and Thienpont1973; Genchi et al., Reference Genchi, Traldi and Manfredi1990). Side-effects of mebendazole such as vomiting and diarrhoea can occur already when therapeutic doses are administered. Moreover, the compound has been associated with hepatotoxicity visible as icterus, depression or anorexia and side-effects commonly occur 1 day until 2 weeks after administration (Polzin et al., Reference Polzin, Stowe, O'Leary, Stevens and Hardy1981; Swanson and Breider, Reference Swanson and Breider1982). In the current case, the dog was closely monitored, and no side-effects of any kind were observed even when mebendazole was given at the increased dose. A thorough surveillance of dogs being treated with this compound seems reasonable, especially if administered off-label. As mebendazole had at least induced an apparent reduction of the number of excreted proglottids, it was decided to increase the dose to 86.2 mg kg−1 BW. This eventually turned out to be effective after the failure of multiple agents as well as mebendazole at a lower dose. However, it cannot be excluded that shedding of proglottids stopped due to reaching of the natural lifetime of the parasite (Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018). Yet, the macroscopic appearance of the proglottids and their motility evidently changed after initiation of mebendazole administration supporting the resistance of this D. caninum isolate to previous anthelmintic treatments and rather ruling out the potential natural termination of infection.
One aspect to consider was the potential of flea infestation as a source of continuous reinfection of the patient. Consequently, the inability to eliminate the parasite could have been mistakenly interpreted as anthelmintic resistance with only temporary elimination of the infection. Yet, reinfection due to continued flea infestation of the dog appeared extremely unlikely as the dog was repeatedly treated against ectoparasites and in accordance with the information provided by the manufacturer the treatments were still exerting their flea-insecticidal activity during the investigations and their follow-up. Moreover, the owners implemented intensive environmental surveillance for potential presence of fleas as well as environmental decontamination, although indications of flea infestation were absent. Flea absence is further corroborated by the fact that the dog remained negative for a follow-up period of more than 3 months after the last anthelmintic treatment as well as for an extended follow-up period of at least 10 months.
Owner compliance is an obviously relevant aspect in the context of suspected drug resistance when the administration of anthelmintics is delegated to the pet owner. This may include anthelmintics that are not administered in the right dose or frequency (Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018) or if the animal, unobserved, expels orally administered drugs. In the present case, owner compliance, involvement, and engagement were exceptionally high and due to the background of the owner as a medical practitioner, correct observation of the case, meticulous documentation as well as appropriate administration of medications were ensured.
Infections with D. caninum in humans are rare, associated with mild clinical signs such as discomfort or gastrointestinal disturbances (Taylor and Zitzmann, Reference Taylor and Zitzmann2011; Portokalidou et al., Reference Portokalidou, Gkentzi, Stamouli, Varvarigou, Marangos, Spiliopoulou and Dimitriou2018), and mainly limited to cases where flea infestations and oral ingestion of fleas are present, e.g. in infants (Chappell et al., Reference Chappell, Enos and Penn1990; Molina et al., Reference Molina, Ogburn and Adegboyega2003; Jesudoss Chelladurai et al., Reference Jesudoss Chelladurai, Kifleyoannes, Scott and Brewer2018). Given the interconnectedness of human and animal health, the emergence of anthelmintic resistance in D. caninum could pose a minimal risk to human health as well.
Conclusions
Future investigations are necessary to understand the extent of potential anthelmintic resistance present in D. caninum infecting dogs and cats, and the mechanisms involved to confer this resistance. Importantly, notifying the occurrence of similar cases with pharmacovigilance authorities will contribute to better data collection. Major challenges are represented by the limited availability of alternative effective compounds and their restricted availability depending on the country.
Data availability statement
All data have been presented and/or are available on request from the authors.
Acknowledgements
We wish to cordially acknowledge Stefania Zanet and Helder Cortez for their help with purchasing and shipping medications from Italy and Portugal, respectively.
Author's contribution
Conceptualization, M. S.; investigation, A. R., A. B., A. W. E. and M. S.; methodology, M. S., A. R. and A. W. E.; resources, M. S. and A. R.; writing – original draft, A. W. E. and M. S.; writing – review and editing, A. R., A. B., A. W. E. and M. S. All authors have read and agreed to the submitted version of the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standards
Informed and written consent for the anthelmintic treatments and the coproscopic analyses was obtained from the animal owner.