Introduction
Major depressive disorders (MDD) represent the second leading cause of disability worldwide due to the deficits in socio-occupational function and the decline in physical health that they produce (McRae, O'Donnell, Loukine, Rancourt, & Pelletier, Reference McRae, O'Donnell, Loukine, Rancourt and Pelletier2016; Whiteford et al., Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine and Vos2013). At present, although a large variety of antidepressants agents (AD) with different mechanisms of action are available to treat this condition, no significant differences in efficacy and safety have been shown between them (Miura et al., Reference Miura, Noma, Furukawa, Mitsuyasu, Tanaka, Stockton and Kanba2014). Moreover, no biochemical, genetic, structural nor phenomenological marker has been proven useful to personalize the selection of an antidepressant (Perlis, Reference Perlis2016).
An alternative way to find the optimal AD for a given patient could be found in the users' opinions and experiences while receiving these drugs. Prior research has shown that inquiry about the subjective experiences of consumers is a valid way to predict adherence and other outcomes in both schizophrenia and mood disorders (for a revision see Strejilevich et al., Reference Strejilevich, Camino, Caravotta, Valerio, Godoy, Gordon and Goldfarb2019). On the other hand, exploring users' experiences and opinions is useful to highlight clinical or adverse effects not routinely evaluated. After conducting a similar study on antipsychotics (Moncrieff, Cohen, & Mason, Reference Moncrieff, Cohen and Mason2009), Goldsmith and Moncrieff (Reference Goldsmith and Moncrieff2011) explored data from the website www.askapatient.com and described the psychological and physical side effects experienced by users of fluoxetine and venlafaxine, reporting that approximately 20% of users had experienced emotional adverse effects such as emotional blunting. These data agree with a growing amount of users' reports and qualitative studies which have shown that AD can produce emotional/behavioral side effects that impact on overall functioning and treatment adherence (for a review see Szmulewicz et al., Reference Szmulewicz, Samamé, Caravotta, Martino, Igoa, Hidalgo-Mazzei and Strejilevich2016). However, to the best of our knowledge, there are no studies that have investigated whether different AD determine different subjective experiences as well as the impact that these may have on the level of satisfaction and adherence with the treatment.
Aims of the study
The objective of this work is to compare the opinions and experiences of individuals who have been exposed to different AD agents. We therefore extracted a random sample of posts from the www.askapatient.com website to explore whether differences in the overall satisfaction and in specific side effects existed between the most frequently used AD agents. Our hypothesis is that the subjective experience of users will differ between individual agents, which will impact on the overall satisfaction reported.
Methods
Data source
We conducted a qualitative and quantitative analysis of the posts on different AD agents from the website www.askapatient.com. This is a website designed to recompile opinions and experiences from users who currently take or have taken a wide range of medications and on which people can record negative and/or positive experiences about each medication and rate them on a scale from 1 (most negative) to 5 (most positive). Respondents are also asked to enter their age, gender, diagnosis, dose, and the length of time they have been taking the drug. No information on concomitant medications is recorded. We have considered these posts analogous to public records since all data on the website are publicly available and anonymous, and posting a comment on a drug does not require respondents to register, although they may disclose their email address. Hence, we judged it ethically acceptable to conduct a passive analysis of the comments without seeking informed consent from their authors (Eysenbach & Till, Reference Eysenbach and Till2001).
Selection of responses
Considering the extensive number of entries, we restricted our search to nine of the most frequently used antidepressants: citalopram, sertraline, paroxetine, escitalopram, fluoxetine, venlafaxine, duloxetine, bupropion, and mirtazapine. Secondly, all comments from www.askapatient.com on the included drugs were numbered consecutively. By simple random sampling, we randomly selected 1000 comments. Finally, we selected the posts that fulfilled the following inclusion criteria: (a) the AD was used in an appropriate dosage range (see online Supplementary Table S1); (b) the AD had to be used for at least of 4 weeks; (c) the indication for the AD had to be reported (i.e. non-missing) and; (d) fewer than 50 posts from that AD agent were already extracted.
Side effects and data extraction
Before reviewing all the entries in the databases, a list with all possible side effects was constructed by consensus between authors. This list included the more typical side effects reported in patients under AD treatment: headache, gastrointestinal symptoms, sexual disturbance, weight changes, sedation, or insomnia as well as subjective side effects. We defined subjective adverse effects following Goldsmith and Moncrieff (Reference Goldsmith and Moncrieff2011), that is, emotional blunting, sedation, and cognitive impairment. We also incorporated to this list emotional hyper-reactivity and irritability on the basis of prior research conducted on AD subjective side effects (Goldsmith & Moncrieff, Reference Goldsmith and Moncrieff2011; Szmulewicz et al., Reference Szmulewicz, Samamé, Caravotta, Martino, Igoa, Hidalgo-Mazzei and Strejilevich2016). Finally, the overall subjective experience to the AD treatment was defined by the overall rating that scales from 1 (most negative) to 5 (most positive).
Two authors (SAS and SC) independently went through the comments initially, to identify recurrent themes (online Supplementary Table S2). This examination was not conducted blind to the drug type. The provisional list of possible effects was used as a guide, but additional effects and experiences were identified during inspection of the comments. The final list was produced by iterative reviews of the data and consensus among authors.
Finally, relevant covariates were extracted for each selected comment: type of AD, age, gender, diagnosis (i.e. major depression, bipolar disorder, anxiety disorders, and pain conditions), time since start of AD treatment, dosage, overall rating, and side-effects reported.
Data analysis
Clinical, pharmacological, and demographic characteristics of included patients are described using proportions, mean and standard deviation (s.d.), or median and interquartile range (IQR) as appropriate. We used linear regression analyses to compare each individual drug and overall ratings, adjusting for potential confounders.
In exploratory analyses, we then computed the crude associations between the presence of specific side effects and the use of either serotoninergic or non-serotoninergic agents by fitting logistic regression models with each side effect as dependent variable and drug class as a regressor. In this analysis, we grouped together selective serotonin reuptake inhibitors (SSRI) and serotonin-norepinephrine reuptake inhibitors (SNRI) because the latter (i.e. venlafaxine and duloxetine) may inhibit the neuronal reuptake of serotonin while at the same time decrease dopamine production (Stahl & Grady, Reference Stahl and Grady2019). Bupropion and mirtazapine were grouped together as they lack any serotonin reuptake effect and increase dopamine production (Arnone, Horder, Cowen, & Harmer, Reference Arnone, Horder, Cowen and Harmer2009; Poyurovsky et al., Reference Poyurovsky, Epshtein, Fuchs, Schneidman, Weizman and Weizman2003). In multivariate analyses, we reported the same odds ratios but adjusted by potential confounders (i.e. age, gender, diagnosis, time under treatment, and dose). In a sensitivity analysis, we repeated our analysis, but we analyzed drug classes as SSRI, SNRI, and dopaminergic agents, separately, using SSRI as the reference category.
All analyses were conducted using R statistical software (version 3.5.2).
Results
A total of 450 posts were included in the present analysis (Fig. 1). Median age was 37 years (IQR: 26.25–49.00), and 29.8% of the sample was comprised by males. In total, 66.7% had a diagnosis of depression, 4.7% had a diagnosis of bipolar, and 51% suffered from anxiety. Median duration of treatment was 210.0 days (IQR: 90.0–775.5 days). Median dose of sertraline was 75.0 (IQR: 50–100), of paroxetine 25.0 (20.0–40.0), of citalopram 25.0 (20.0–40.0), of escitalopram 10.0 (IQR: 10.0–20.0), and of fluoxetine 22.5 (20.0–40.0) (Table 1). As expected, sexual disturbances were reported more frequently by users of SSRI and SNRI while very few users of mirtazapine and bupropion reported this side effect. Sedation was more frequently reported by users of mirtazapine and fluoxetine. Conversely, bupropion users more frequently reported insomnia (Table 1).
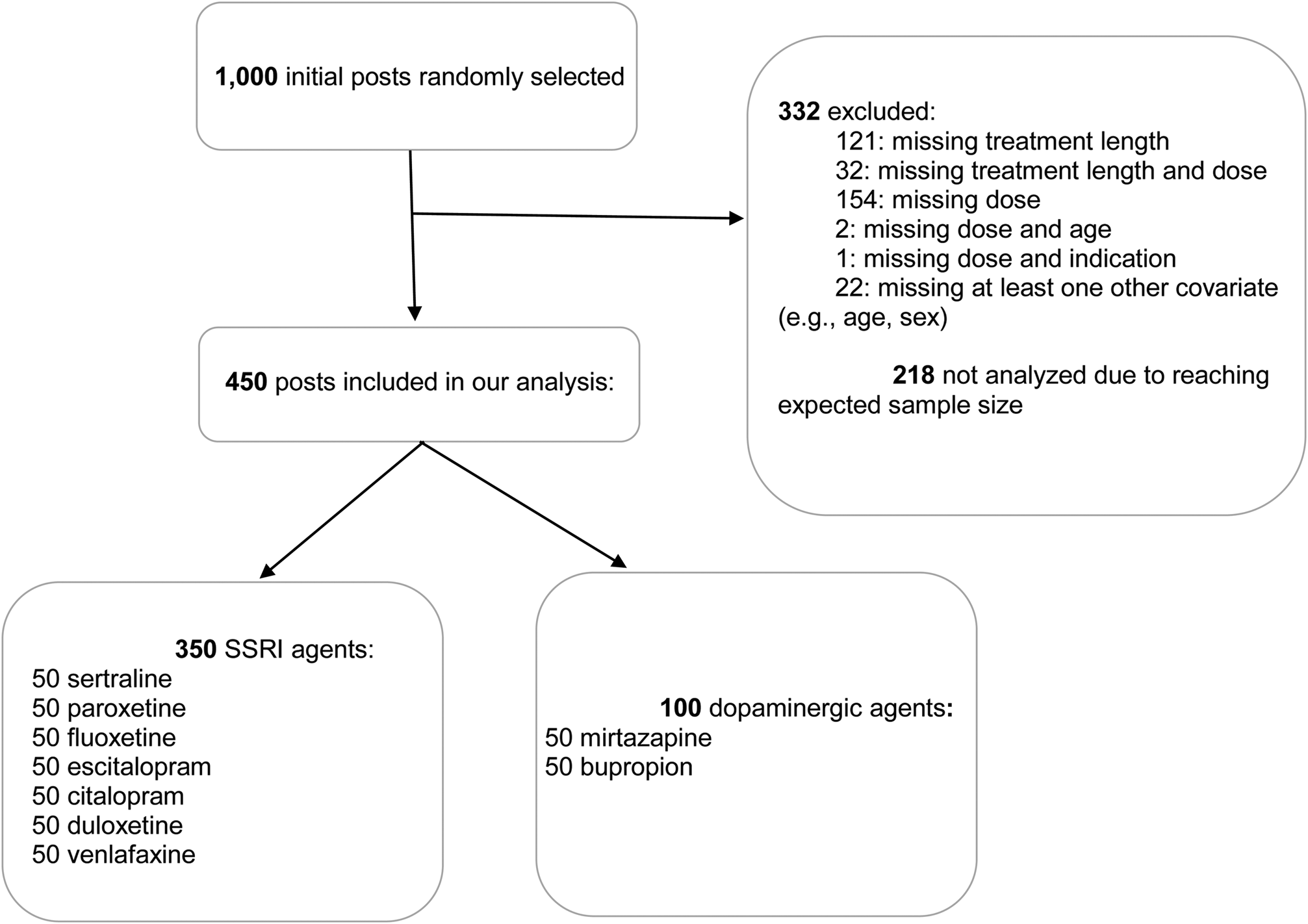
Fig. 1. Flow chart of selected posts.
Table 1. Characteristics of posts included

s.d., standard deviation; OCD, obsessive compulsive disorder; MDD, major depressive diagnosis; N/A, not applicable.
Emotional adverse effects
A total of 189 patients reported the presence of any emotional adverse effect in our sample, for a point prevalence of 42.0%. The most frequently reported emotional adverse effect was emotional blunting (18.0%) followed by emotional hyper-reactivity (16.0%) and withdrawal (14.7%). Emotional blunting was more frequently reported by users of SSRI (paroxetine, sertraline, and fluoxetine) and less frequently by users of bupropion and mirtazapine (Table 1).
Differences in the individual profile of emotional adverse effects between AD drugs emerged. For example, paroxetine was associated with emotional blunting, withdrawal, and irritability; bupropion with emotional hyper-reactivity; venlafaxine with withdrawal and irritability; and mirtazapine with withdrawal and hyper-reactivity, although to a lesser extent (Table 1). Finally, when we explored predictors of emotional adverse events, we found that bipolar disorder patients were more likely to report hyper-reactivity, younger patients were more likely to report emotional blunting, and cognitive disturbances were more likely to be reported by patients with pain conditions and with bipolar disorder (online Supplementary Table S3).
Overall rating satisfaction
Overall, the agent with a higher mean satisfaction was bupropion (3.8, s.d.: 1.3), followed by citalopram (3.7, s.d.: 1.3) and venlafaxine (3.7, s.d.: 1.3). The stronger differences between individual agents in terms of their ratings were between bupropion (3.82) and duloxetine (mean rating: 2.98) for a mean difference of 0.84 points (95% CI −0.07 to 1.76) and between bupropion and paroxetine (3.06), for a mean difference of 0.76 (95% CI −0.16 to 1.67) (Table 1). Predictors of better treatment satisfaction were presenting an anxiety disorder and a longer treatment duration. Predictors of a worse treatment satisfaction were presence of emotional (emotional blunting, emotional hyper-reactivity, suicidality, irritability, and withdrawal) and cognitive adverse events (Table 2).
Table 2. Univariate predictors of antidepressant-overall rating

CI, confidence interval; predictors with an associated p value below 0.05 were bolded.
The adverse events that showed a greater association with the overall rating were suicidality, irritability, emotional blunting, cognitive disturbances, and withdrawal symptoms (Table 2 and Fig. 2).

Fig. 2. Bar plot demonstrating the proportion of users of antidepressants reporting a specific side-effect to the website “www.askapatient.com”.
Exploratory analysis: serotoninergic v. non-serotoninergic agents
The use of non-serotoninergic agents was associated with a slightly higher overall rating (β = 0.42, 95% CI 0.01–0.75) after adjusting for potential confounding factors, such as age; gender; self-reported diagnosis of pain, depression, or anxiety; bipolar disorder; duration of antidepressant use; and dosage.
We then inspected the association between drug class (serotoninergic v. non-serotoninergic) and each of the complaints that predicted overall ratings. We found a strong inverse association between the use of non-serotoninergic agents and self-reported emotional blunting (OR 0.27, 95% CI 0.11–0.58), but no association for irritability (OR 0.80, 95% CI 0.25–2.12), suicidality (OR 0.21, 95% CI 0.01–1.10), withdrawal (OR 0.46, 95% CI 0.20–1.08), or cognitive complaints (OR 1.21, 95% CI 0.57–2.56), after adjusting for potential confounders.
Sensitivity analysis
We found that SNRI agents had a slightly lower overall satisfaction rating as compared to SSRI (−0.47, 95% CI −1.02 to 0.07), while non-serotoninergic agents had a significantly higher rating as compared to SSRI agents (0.44, 95% CI 0.09–0.79). Non-serotoninergic agents also had a higher overall rating as compared to SNRI (0.92, 95% CI 0.33–1.51). Similarly, while a non-significant increase report in emotional blunting could be observed with SNRI as compared to SSRI agents (OR 1.38, 95% CI 0.58–3.30), we found a significantly decreased odds of reporting emotional blunting in users of non-serotoninergic agents as compared to SSRI (OR 0.25, 95% CI 0.11–0.59) and SNRI (0.18, 95% CI 0.06–0.56).
Discussion
The main goal of this work is to describe the individual profiles of adverse effects produced by the most widely used AD agents, as reported by their users. Especially, we focus in describing the different profiles of emotional adverse effects. These are often not included in patient–doctor conversations about which drug to initiate and were found to be highly reported in our sample (42%), in keeping with prior reports on this topic (Goldsmith & Moncrieff, Reference Goldsmith and Moncrieff2011). In fact, we found that emotional blunting (the most frequently reported emotional adverse effect) was strongly associated with the overall rating of satisfaction of the users with their treatment. We found that paroxetine, sertraline, and fluoxetine (i.e. serotoninergic agents) were more likely to produce emotional blunting than bupropion. Irritability was less frequently reported (6% of the sample), but also strongly associated with treatment satisfaction. Paroxetine, venlafaxine, and bupropion were more strongly linked to the production of this emotional adverse effect. Overall, mirtazapine was associated with a low frequency of adverse emotional effects, as compared to the other drugs. Finally, in exploratory analyses, we found that users would better consider agents with a mainly non-serotoninergic mechanism of action as compared to those with a serotonergic one, and was robust to sensitivity analysis changing the drug classification. In fact, individual differences in overall rating were stronger when comparing SNRI agents (duloxetine and venlafaxine) with bupropion, after adjusting for potential confounders.
Emotional blunting (or emotional flattening) is a side effect consistently described by patients receiving AD treatment. It is described as the experience to feel a reduction (or absence) in emotional responding for both, rewarding and aversive stimuli. This experience is also described as being a core part of the apathy syndrome (Mortby, Maercker, & Fortsmeier, Reference Mortby, Maercker and Fortsmeier2012). It can range in its intensity from a minimum (often experienced as positive by those people treated by with anxiety symptoms) to such an intensity that leads people into feeling like robots, zombies, or emotionally dead (Price, Cole, & Goodwin, Reference Price, Cole and Goodwin2009). Although the capacity of some AD to produce emotional blunting was reported since the approval of the first AD (Kramer, Klein, & Fink, Reference Kramer, Klein and Fink1961), their reporting significantly increased with the dissemination of the use of modern serotonergic AD (Kramer, Reference Kramer1994). In recent years, a series of exploratory studies have shown that this emotional side effect has negative impact on overall functioning, treatment adherence, and decision-making capacity and it has been positively correlated with the incidence of suicidal and self-harming attempts (for a review see Szmulewicz et al., Reference Szmulewicz, Samamé, Caravotta, Martino, Igoa, Hidalgo-Mazzei and Strejilevich2016). In addition, a series of studies which have explored AD impact on emotional processing have found that emotional blunting would be a specific effect of those AD with a predominantly serotonergic mechanism of action (Pringle, McCabe, Cowen, & Harmer, Reference Pringle, McCabe, Cowen and Harmer2013). While these agents produce a reduction in the emotional response to positive and negative emotional stimuli, dopaminergic agents only reduce the emotional response to adverse stimuli, preserving the response to rewarding ones (Arnone et al., Reference Arnone, Horder, Cowen and Harmer2009; Harmer, Hill, Taylor, Cowen, & Goodwin, Reference Harmer, Hill, Taylor, Cowen and Goodwin2003; Harmer et al., Reference Harmer, de Bodinat, Dawson, Dourish, Waldenmaier, Adams and Goodwin2011). These different effects on emotional processing would be mediated by a down-regulation of dopamine turn-over in circuits consistently associated with emotional blunting and apathy due to the chronic elevation of serotonin levels in the nucleus accumbens secondary to 5HT2C agonism of SAD exposition (Levy & Dubois, Reference Levy and Dubois2006; Stahl, Reference Stahl2013).
Our results on withdrawal symptoms deserve special comment. In this work, as well as in previous studies that have explored the opinions and experiences of AD users, withdrawal symptoms are generally among the more prevalent complaints (Moncrieff et al., Reference Moncrieff, Cohen and Mason2009; Price et al., Reference Price, Cole and Goodwin2009; Stockmann, Odegbaro, Timimi, & Moncrieff, Reference Stockmann, Odegbaro, Timimi and Moncrieff2018). The withdrawal symptoms of AD have been described since the beginning of their use, but only recently has the clinical complexity of their presentation been understood (Fava et al., Reference Fava, Benasi, Lucente, Offidani, Cosci and Guid2018) and the difficulty in withdrawing them has been considered as another factor to take into account at the time to select an AD (Iacobucci, Reference Iacobucci2019). In this work, we found a lower report of withdrawal symptoms with bupropion as compared to the rest of the serotoninergic AD and also mirtazapine (Stockmann et al., Reference Stockmann, Odegbaro, Timimi and Moncrieff2018). Future papers should explore this issue with specific study design.
Finally, it is worth noting that AD users, in general, reported a higher level of satisfaction with these agents (i.e. a mean of 3.4 in a scale from 0 to 5) suggesting the overall positive impact these agents had on their subjective experience. This agrees with the results of a survey among AD users in New Zealand in which 54% of participants reported a positive of antidepressants' impact in their lives, 28% ‘mixed’ experiences, and only 16%, negative experiences (Gibson, Cartwright, & Read, Reference Gibson, Cartwright and Read2016).
This study has several limitations that deserve to be considered. First, the data here analyzed come from a website on which people posting spontaneously their experiences. Therefore, these data could represent a non-random sample of all AD users (i.e. comprised mostly by people who had bad experience with these drugs). However, as we noted above, the satisfaction ratings indicate that over a half of these users reported a positive consideration about AD treatment. Second, all information given by patients was self-reported, and hence it remains plausible that individuals may misinterpret symptoms of their disease as subjective side effects. In a similar vein, we were unable to discriminate if some of the reported side effects reported could have been precipitated by another drug that the individuals may have been receiving at the same time. Third, we were unable to control by disease severity characteristics. For example, it is possible that non-serotoninergic agents were prescribed preferentially to patients with a lower disease severity and thus more likely to be helped by these agents. However, we adjusted for some proxies of disease severity available such as dosage of the AD, length of treatment, and diagnosis. Fourth, because of our inclusion criterion requiring at least 4 weeks, it might be that some of the early side effects, such as gastrointestinal symptoms, may be underreported. Fifth, we did not perform a formal sample size calculation and hence some of our results might be prone to type II error. A final limitation is that emotional blunting could be a feature of the underlying depression. If patients with mirtazapine or bupropion were less frequently receiving these drugs due to depression, this might explain the finding of a lower incidence of this subjective side effect. Although we adjusted for the clinical indication in our adjusted analyses (i.e. pain diagnosis, depression, bipolar disorder, and anxiety disorder), this variable was self-reported and it might not be accurate. Future studies using clinically-validated diagnoses to confirm our findings are warranted.
Beyond these limitations, the results of the present work show that inquiring about users' experiences could contribute to open new pathways in order to achieve a better approach for AD selection. These users have shown a preference for non-serotoninergic agents, in part due to their lower propensity to produce emotional blunting, predilection that goes in the opposite direction to the most frequent clinical behavior in which in more than 70% of the choices of a first antidepressant fall on an SSRI (Bauer et al., Reference Bauer, Monz, Montejo, Quail, Dantchev, Demyttenaere and Tylee2008).
It is worth noting that although this is a consideration that emerged from the experiences of users with AD, it is consistent with the prevailing hypotheses regarding the mechanisms of action of these compounds at the cellular, psychological, and behavioral levels (Harmer et al., Reference Harmer, Hill, Taylor, Cowen and Goodwin2003). Finally, the results of this work highlight the need to pay more attention to the adverse psychological and emotional effects of AD when evaluating its effectiveness and acceptability. Future research should incorporate systematic assessments of subjective side-effects and subjective experience of the users of AD agents in clinical samples and in randomized controlled trials.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291722000678.
Data
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of interest
None.







