Introduction
Somatic symptom and related disorders (SSRD) are characterized by somatic symptoms that are associated with significant distress and impairment (American Psychiatric Association (APA), 2013). SSRD constitutes a new category in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5 (APA, 2013) and replaces the previous diagnostic classification of somatoform disorders that was used in the DSM-IV-TR (APA, 2000). SSRD differs from somatoform disorders in the number of disorders and subcategories. The category SSRD consists of illness anxiety disorder, conversion disorder, factitious disorder, somatic symptom disorder, psychological factors affecting other medical conditions, unspecified somatic symptom and related disorder, and other specified somatic symptom and related disorder (APA, 2013). The criterion of somatoform disorder according to the DSM-IV-TR, which stated that physical symptoms had to be medically unexplainable was disposed of because it was hard to determine whether or not a symptom in fact is medically unexplainable (Barsky, Reference Barsky2016). Therefore, several suggestions were made (van der Feltz-Cornelis & van Balkom, Reference Van der Feltz-Cornelis and van Balkom2010) and the focus changed toward coping with physical symptoms rather than searching for their cause (Rief & Martin, Reference Rief and Martin2014; Barsky, Reference Barsky2016).
Because of its recent introduction and the conceptual differences with somatoform disorders, studies in samples of patients with SSRD are scarce, and previous studies that focused on somatoform disorders are not necessarily generalizable to the SSRD population since physical symptoms do not have to be medically unexplainable using the new DSM-5 classification. As a result, little is known about patients with SSRD, in particular regarding neurocognitive functioning.
Previous research has shown that neurocognitive dysfunctioning of patients with late-life somatic symptom disorder is common (Inamura et al. Reference Inamura, Shinagawa, Nagata, Tagai, Nukariya and Nakayama2015). However, the details regarding neurocognitive dysfunctioning of patients with SSRD are unknown. Since no studies are currently available regarding the neurocognitive profile of adults with SSRD, a brief summary of neurocognitive profiles of somatoform disorders is given. In particular, results from studies on neurocognitive dysfunctioning of patients with somatoform disorders suggest impaired (working) memory (Grace et al. Reference Grace, Nielson, Hopkins and Berg1999; Niemi et al. Reference Niemi, Portin, Aalto, Hakala and Karlsson2002; Luerding et al. Reference Luerding, Weigand, Bogdahn and Schmidt-Wilcke2008; Al-Adawi et al. Reference Al-Adawi, Al-Zakwani, Obeid and Zaidan2010; Demir et al. Reference Demir, Celikel, Taycan and Etikan2013; Brown et al. Reference Brown, Nicholson, Aybek, Kanaan and David2014), executive functioning (Al-Adawi et al. Reference Al-Adawi, Al-Zakwani, Obeid and Zaidan2010; Demir et al. Reference Demir, Celikel, Taycan and Etikan2013; Brown et al. Reference Brown, Nicholson, Aybek, Kanaan and David2014), attention and concentration (Grace et al. Reference Grace, Nielson, Hopkins and Berg1999; Niemi et al. Reference Niemi, Portin, Aalto, Hakala and Karlsson2002; Demir et al. Reference Demir, Celikel, Taycan and Etikan2013), and visuospatial functioning (Niemi et al. Reference Niemi, Portin, Aalto, Hakala and Karlsson2002; Demir et al. Reference Demir, Celikel, Taycan and Etikan2013). The pattern of results is inconsistent and most studies have not adjusted for important confounding variables such as comorbid depression, included small sample sizes, or focused on only a limited number of neurocognitive domains. In addition, studies did not include symptom validity tests in the neurocognitive test battery, such as a test assessing the presence of malingering. Therefore, results of previous studies regarding neurocognitive dysfunctioning of patients with somatoform disorders should be interpreted cautiously.
Research has shown that the prevalence of comorbid depression in patients with medically unexplained physical symptoms (13.5%), medically explained physical symptoms (7.4%), and medically explained combined with unexplained physical symptoms (10.9%) is higher than the prevalence of depression in patients with no physical symptoms (5.1%) (van der Sluijs et al. Reference Van der Sluijs, Ten Have, Rijnders, van Marwijk, de Graaf and van der Feltz-Cornelis2015). However, the influence of comorbid depression on neurocognitive dysfunctioning of patients with SSRD has not been explored yet.
Patients with a depressive disorder show increased neurocognitive impairment across multiple domains such as attention (Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014), information processing speed (Tsourtos et al. Reference Tsourtos, Thompson and Stough2002; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Bennabi et al. Reference Bennabi, Vandel, Papaxanthis, Pozzo and Haffen2013), memory (Murrough et al. Reference Murrough, Iacoviello, Neumeister, Charney and Iosifescu2011; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014), and executive functioning (Murrough et al. Reference Murrough, Iacoviello, Neumeister, Charney and Iosifescu2011; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Snyder, Reference Snyder2013; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014). The extent to which neurocognitive dysfunctioning has been reported to be proportional to the severity of the depressive disorder r(Wang et al. Reference Wang, Halvorsen, Sundet, Steffensen, Holte and Waterloo2006; Castaneda et al. Reference Castaneda, Tuulio-Henriksson, Marttunen, Suvisaari and Lonnqvist2008). Anxiety is also associated with neurocognitive dysfunctioning (Castaneda et al. Reference Castaneda, Tuulio-Henriksson, Marttunen, Suvisaari and Lonnqvist2008; Tempesta et al. Reference Tempesta, Mazza, Serroni, Moschetta, Di Giannantonio, Ferrara and De Berardis2013), such as impairment of executive functioning, memory, attention, and learning (De Geus et al. Reference De Geus, Denys, Sitskoorn and Westenberg2007; Harkin & Kessler, Reference Harkin and Kessler2011; Polak et al. Reference Polak, Witteveen, Reitsma and Olff2012; Tempesta et al. Reference Tempesta, Mazza, Iaria, De Gennaro and Ferrara2012).
However, the association of comorbid depression and anxiety on neurocognitive functioning of patients with SSRD has not been explored yet. If SSRD, depression and anxiety independently would have a negative influence on neurocognitive functioning, then it is plausible that comorbid depression and anxiety in patients with SSRD might impair neurocognitive dysfunctioning. Hence, a comparison between the neurocognitive profile of SSRD patients with and without comorbid depression and anxiety would be of substantial clinical relevance. It may not only increase insight in the disorder but might also lead to new treatment options, which might increase effectivity and lead to a faster reduction of symptoms and better coping with SSRD. However, until now, studies exploring cognitive dysfunctioning and the impact of comorbid depression and anxiety of patients with SSRD are lacking.
This study had two objectives. The first objective was to establish the prevalence and severity of neurocognitive dysfunctioning, comorbid depression and comorbid anxiety disorder in patients with SSRD. We hypothesized that patients with SSRD show extensive neurocognitive dysfunctioning within the domains of attention and concentration, information processing speed, memory, and executive functioning compared to the most recent norms. The second objective was to evaluate whether comorbid depression and anxiety in SSRD adversely affect neurocognitive functioning. We hypothesized that neurocognitive dysfunctioning is poorer for patients suffering from comorbid depression (SSRD+D) and comorbid anxiety (SSRD+A) than for patients without comorbid depression (SSRD−D) and anxiety (SSRD−A), respectively. Specifically, we expected that patients with SSRD+D and patients with SSRD+A have more severe impairment in the domains of attention and concentration, information processing speed, memory, and executive functioning.
Method
Study design
A cross-sectional design was used to address the study aims.
Setting and participants
Consecutive outpatients (N = 250) older than 18 years, referred to Clinical Centre of Excellence for Body, Mind and Health (Dutch abbreviation: CLGG), at specialty mental health institution GGz Breburg, Tilburg, the Netherlands, participated in this study. For all patients referred to CLGG, we evaluated the inclusion and exclusion criteria before intake. Patients were excluded if they (a) were engaged in personal or professional injury procedures, (b) had an intelligence quotient (IQ) below 80, (c) had an active suicide risk (threatening) or (d) suffered from substance abuse. Patients referred to CLGG filled out questionnaires as part of routine clinical care [i.e., Routine Outcome Monitoring (ROM)] before intake at CLGG (Van der Feltz-Cornelis et al. Reference Van der Feltz-Cornelis, Andrea, Kessels, Duivenvoorden, Biemans and Metz2014). The standard intake procedure at CLGG includes a neuropsychological assessment (NPA) and a Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998) of which the data are used in this study.
This study was approved by the Commission of Scientific Research of GGz Breburg (CWO 2014–16). In the intake letter, patients of CLGG were asked for informed consent to participate in scientific research. No consequences for treatment options were present if patients decided not to participate. We excluded patients from this study who did not agree to the use of their data for scientific purposes.
Variables
Somatic symptom and related disorder (SSRD)
SSRD classification was established as follows. The psychiatrists at CLGG diagnosed SSRD DSM-5 classifications based on a checklist administered after psychiatric examination. The classifications were checked later with MINI classifications for somatoform disorders after the MINI interview held by trained psychologists. Discrepancies between interview and symptom-check diagnoses were settled by consensus.
Demographic variables
During intake, we obtained demographic variables such as age, sex and education. Educational level was classified using the method described by Verhage (Reference Verhage1964) and further divided into low level of education (Verhage 1–4), the average level of education (Verhage 5), and high-educated (Verhage 6–7). We used the Dutch version of the National Adult Reading Test (Schmand et al. Reference Schmand, Lindeboom and Van Harskamp1992) to assess verbal premorbid intelligence.
Neuropsychological assessment (NPA)
We administered a standardized comprehensive NPA covering a broad range of neurocognitive domains. NPAs were administered by bachelor's-level clinicians and (neuro)psychologists with extensive training. The NPAs were administered under the supervision of a mental health psychologist. Table 1 displays the neurocognitive tests that were used for assessing the neurocognitive domains.
Table 1. Neurocognitive domains and tests used in the NPA

TMT, Trail Making Test; WAIS-IV, Wechsler Adult Intelligence Scale – fourth edition; RAVLT, Rey Auditory Verbal Learning Test; ROCFT, Rey Osterrieth Complex Figure Test; BADS, Behavioural Assessment of the Dysexecutive Syndrome.
More specifically, we used the d2 test (Brickenkamp, Reference Brickenkamp2002) to measure sustained attention. The d2 test is considered a valid test (Bates & Lemay, Reference Bates and Lemay2004). We measured divided attention using the Trail Making Test (TMT) (Reitan, Reference Reitan1992) B-version. The TMT-B score was calculated as the proportion of the completion time for TMT-A, and is a measure for divided attention (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012). The subtest Digit Span from the Wechsler Adult Intelligence Scale (WAIS)-IV was used to assess working memory (Wechsler, Reference Wechsler2014). We used the delayed test score of the Dutch translation of the Rey Auditory Verbal Test (RAVLT) (Saan & Deelman, Reference Saan and Deelman1986) to measure verbal memory. We used the delayed recall score of the Rey Osterrieth Complex Figure Test (ROCFT) (Osterrieth, Reference Osterrieth1944) to assess visual memory (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012). We assessed information processing speed using the subtest Coding from the WAIS-IV (Wechsler, Reference Wechsler2014). Furthermore, we used three tests to assess several domains within executive functioning. We used the Zoo map and the Rule Shift Cards of the Behavioural Assessment of the Dysexecutive Syndrome (BADS) to assess planning and mental flexibility (Wilson et al. Reference Wilson, Alderman, Burgess, Emslie and Evans1996), respectively. We used the ‘N’ and ‘A’ test to assess phonological verbal fluency (Deelman et al. Reference Deelman, Koning-Haanstra, Liebrand and van der Burg1981).
We used raw test scores for analyses and compared the scores on the neuropsychological tests to the most recent norms of the tests (taking into account sex, age, and education) for quantitative description of neurocognitive dysfunctioning. For each neuropsychological test, the norm scores available in the test manuals were used except for the TMT-B and the RAVLT for which we used the norms provided by Schmand et al. (Reference Schmand, Houx and de Koning2012). We divided patients’ performance into three groups: no neurocognitive dysfunctioning (larger than or equal to the 20th percentile of the normal distribution), deficit (larger than or equal to the 2.4th percentile and smaller than the 20th percentile of the normal distribution), and disorder (smaller than the 2.4th percentile of the normal distribution) (Lezak et al. Reference Lezak, Howieson, Bigler and Tranel2012).
Before administering the NPA, we explored malingering using the Test of Memory Malingering (TOMM) (Tombaugh, Reference Tombaugh1996). If the TOMM raises suspicion of malingering (TOMM ⩽ 45 on trial 1 and/or trial 2) (O'Bryant et al. Reference O'Bryant, Gavett, McCaffrey, O'Jile, Huerkamp, Smitherman and Humphreys2008; Denning, Reference Denning2012), the importance of motivation was stressed and discussed with the patient. After a break, the Amsterdam Short-Term Memory Test (Dutch abbreviation: AKTG) (Schmand & Lindeboom, Reference Schmand and Lindeboom2005) was used to further assess malingering. If patients scored positive on the AKTG as well (AKTG < 85; i.e., possible malingering), the NPA was discontinued and patients were excluded from this study.
A symptom validity task was completed by 165 patients to rule out bias related to malingering. Twelve patients displayed signs of malingering and did not complete the NPA. Demographic characteristics (age, sex, and educational level) and baseline symptom severity [Patient Health Questionnaire-9 (PHQ-9) and General Anxiety Disorder questionnaire (GAD-7)] did not significantly differ between patients who were suspected of malingering and patients who were not suspected of malingering.
Depression and anxiety
The self-report scale PHQ-9 (Kroenke et al. Reference Kroenke, Spitzer and Williams2001) was used to measure depression. The PHQ-9 has good psychometric properties (Kroenke et al. Reference Kroenke, Spitzer and Williams2001; Kocalevent et al. Reference Kocalevent, Hinz and Brähler2013), with Cronbach's alpha equal to 0.89 and sensitivity and specificity both of 88%. A cut-off of 10 or higher is advised for assessing moderate levels of depression (Kroenke et al. Reference Kroenke, Spitzer and Williams2002).
We used the GAD-7 (Spitzer et al. Reference Spitzer, Kroenke, Williams and Löwe2006) to measure anxiety. The GAD-7 is a 7-item self-report questionnaire that measures symptoms of anxiety during the last two weeks. The GAD-7 has good psychometric properties (Spitzer et al. Reference Spitzer, Kroenke, Williams and Löwe2006; Löwe et al. Reference Löwe, Decker, Müller, Brähler, Schellberg, Herzog and Herzberg2008), with a Cronbach's alpha equal to 0.92 and with sensitivity and specificity of 89% and 82%, respectively. A cut-off of 10 or higher is advised for assessing moderate levels of anxiety (Spitzer et al. Reference Spitzer, Kroenke, Williams and Löwe2006). A recent report has demonstrated the validity of combining the PHQ-9 and GAD-7 as a single measure for jointly assessing two of the most common psychological conditions in patients with somatic symptoms (Kroenke et al. Reference Kroenke, Wu, Yu, Bair, Kean, Stump and Monahan2016).
Statistical methods
We explored the presence of neurocognitive dysfunctioning of patients with SSRD using the percentages of patients with neurocognitive impairment in the neurocognitive domains using the operationalizations as described in the section Variables. Analyses showed that the scores on the subtests of the BADS were poorly distributed (i.e., only 23.0% of the patients scored below 4 on the Rule Shift Cards). Therefore, we decided to exclude these measures from further analyses. Variables that were not normally distributed were log-transformed.
We explored the association of comorbid depression and comorbid anxiety with neurocognitive functioning separately. Associations between continuous depression and anxiety scores with neurocognitive performance were examined using correlation and multiple regression analyses. In particular, we first obtained the bivariate correlations between neurocognitive dysfunctioning with the PHQ-9 and GAD-7 scores. Second, we used regression analyses to study the relationships between neurocognitive functioning and depression, and neurocognitive functioning and anxiety, while controlling for age, sex, and education level (i.e., high or low educational level) using regression analyses, for assessing the relationship between the PHQ-9 and GAD-7 scores with neurocognitive domains. For these analyses, educational level was dichotomized into a low level of education (Verhage 1–5) and high level of education (Verhage 6–7).
We also used a categorical operationalization of depression and anxiety in which patients were categorized into two clinical groups. In particular, the SSRD+D group was defined as patients with SSRD and a PHQ-9 score ⩾ 10 and the SSRD-D group as patients with SSRD and a PHQ-9 score < 10. The SSRD+A group was defined as patients with SSRD and a GAD-7 ⩾ 10 and the SSRD-A group as patients with SSRD and a GAD-7 < 10. Differences between groups with regard to demographic characteristics and questionnaire scores between SSRD+D v. SSRD-D and SSRD+A v. SSRD+A were examined using independent t tests (for continuous variables) and χ2 tests (for categorical variables). We conducted a sensitivity analysis to compare patients who were suspected of malingering v. patients who were not with regard to demographic and baseline characteristics.
We used multivariate analysis of variance (MANOVA) to compare the neurocognitive profile of patients with SSRD+D v. SSRD-D and patients with SSRD+A v. SSRD−A. Subsequently, differences between neurocognitive domains of patients with SSRD+D v. SSRD−D and patients with SSRD+A v. SSRD−A were considered separately. These analyses were also adjusted for age, sex, and education level. Differences between SSRD+D v. SSRD−D and SSRD+A v. SSRD-A with respect to percentages of no neurocognitive impairments, deficits, and disorders were explored by means of χ2 tests and Fisher's exact tests in case any of the cells have a frequency of less than five. We used the Statistical Package for the Social Sciences version 22.0 (IBM Corporation, 2011) for all analyses.
Results
Participants
Table 2 gives an overview of the demographic characteristics of the total sample and the sample stratified for depression and stratified for anxiety. Two hundred and one patients were included in the analyses (see Fig. 1 for a flowchart). The mean age was 43 [Standard deviation (s.d.) = 13] years and 62% were female. Comorbid depression was observed in 75.1% of the sample [mean score on the PHQ-9, Mean (M) = 14.3, S.D. = 6.0]. Comorbid anxiety was found in 65.7% of the sample (mean score on the GAD-7, M = 11.6, S.D. = 5.5). Depression and anxiety scores were significantly correlated (r = 0.73, p <0.001). 122 patients (60.7%) suffered from both depression and anxiety whereas (19.9%) did not meet criteria for either depression or anxiety, based on the PHQ-9 and GAD-7 scores.
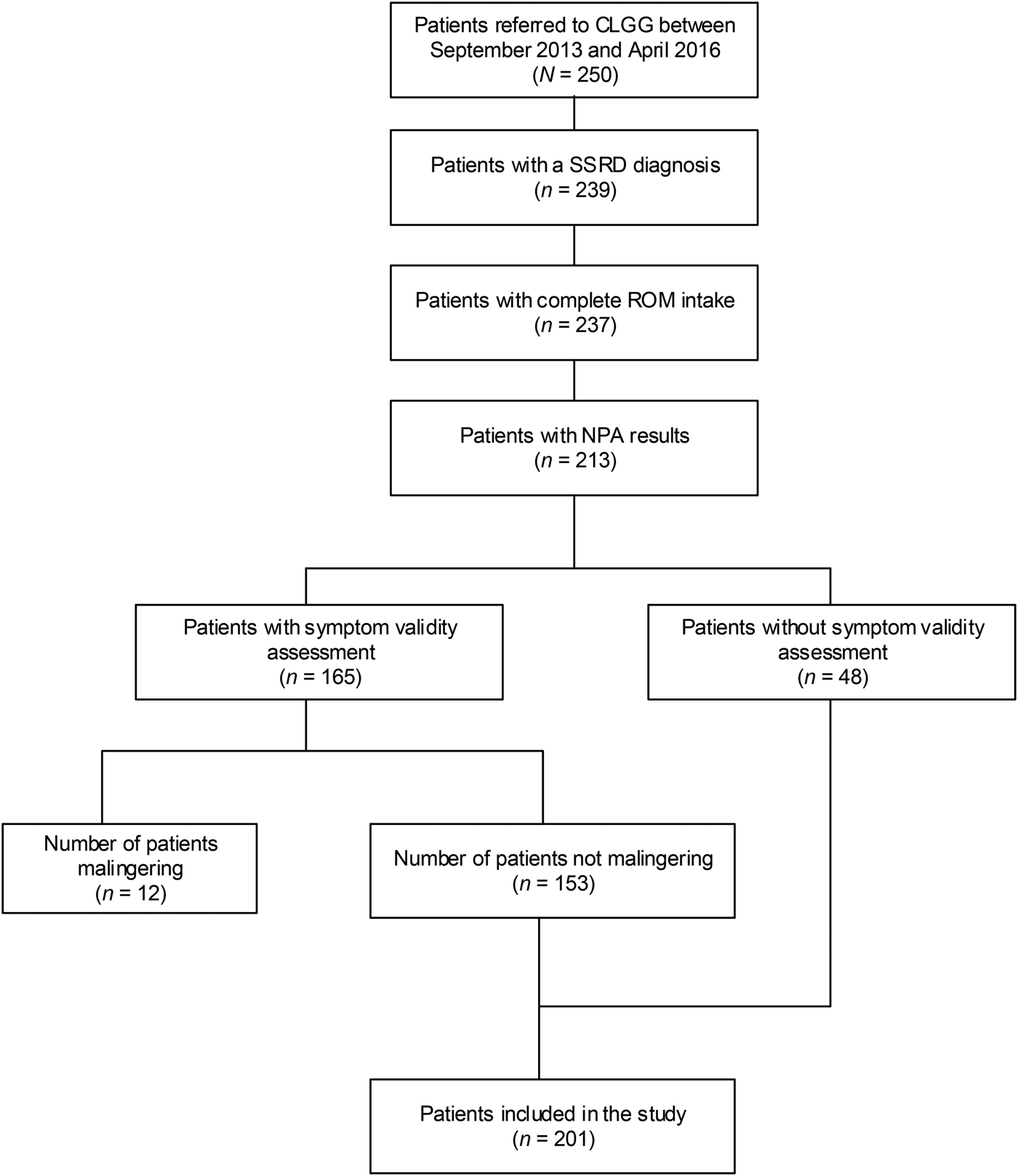
Fig. 1. Flowchart of patients included in this study. CLGG, Dutch abbreviations: Clinical Centre of Excellence for Body, Mind, and Health; SSRD, somatic symptom and related disorders; ROM, routine outcome monitoring; NPA, neuropsychological assessment.
Table 2. Sample descriptive statistics of the total sample of somatic symptom and related disorders (SSRD) and stratified for comorbid depression and anxiety

PHQ-9, Patient Health Questionnaire; GAD-7, Generalized Anxiety Disorder. For depression; a cutoff of 10 or higher on the PHQ-9 was used, and for anxiety; a cut-off of 10 on the GAD-7 was used.
a Cohen's d.
b χ2 tests.
c Cramer's V.
Note. Means (M) and standard deviations (s.d.) are presented for the continuous variables and the number (n) and percentage of patients (%) is presented for the categorical variables.
Demographic characteristics did not differ significantly between patients with depression and patients without depression. Patients with anxiety were significantly younger [t(199) = 2.36, p = 0.02, d = −0.36]. Furthermore, we assessed the premorbid IQ of 185 patients and found a mean IQ of 102 (ranges 72–127). Seven patients had an IQ below 80 and 10 patients had an IQ ranging from 80 to 87. We performed an additional sensitivity analysis to assess differences regarding demographic characteristics and patients with an IQ below 80 were older (M = 55.3, S.D. = 16.4) compared to patients with an IQ higher than 80 (M = 42.0, S.D. = 16.6) and these results differed significantly [t(183) = 2.71, p = 0.007, d = −1.04]. No significant differences were found regarding gender, and the mean scores on the PHQ-9 and GAD-7.
Neurocognitive dysfunctioning of SSRD patients compared to normative data
Table 3 (column 2) describes the neurocognitive functioning of patients with SSRD compared with normative data. Both deficits and clinically impaired neurocognitive disorders were prevalent among patients with SSRD, particularly regarding sustained attention, information processing speed, and working memory. Specifically, 67 (37%) patients had a deficit and 13 (7%) had a disorder with respect to sustained attention. With regard to divided attention, 32 (19%) patients had a deficit and 16 (10%) had a disorder. 67 (35%) patients suffered from a deficit and 23 (12%) from a disorder with respect to information processing speed. A total of 67 (34%) patients had a deficit and 20 (10%) had a disorder within working memory. With regard to verbal memory, 57 (29%) patients had a deficit and 25 (13%) had a disorder. A total of 45 (22%) suffered from a deficit and 37 (20%) from a disorder with respect to visual memory. A total of 12 (6%) patients had a deficit and 2 (1%) had a disorder with respect to planning. With regard to mental flexibility, 6 (3%) patients had a deficit and 5 (3%) had a disorder. 69 (36%) suffered from a deficit with respect to phonological verbal fluency.
Table 3. Neurocognitive functioning of the study sample (N = 201) of somatic symptom and related disorders and stratified for comorbid depression and for comorbid anxiety

Note. Not all 201 patients completed every test; sample size varied between n = 168 and n = 197. The p values are given for the χ2 test and Fisher's exact test.
a Fisher's exact tests were used in the analysis of contingency tables because of violation of the minimum expected cell frequency.
b Differences between patients with and without comorbid depression/anxiety were tested using χ2 tests.
Association of comorbid depression and comorbid anxiety with neurocognitive dysfunctioning
Table 4 shows the zero-order correlations between depression and anxiety scores and neurocognitive measures as well as the regression coefficients from the regression analyses (adjusted for sex, age, and education). The total score of the PHQ-9 significantly correlated with information processing speed (r = −0.17, p = 0.030) and phonological verbal fluency (r = −0.17, p = 0.025), suggesting that a higher depression score was associated with impaired neurocognitive performance within these domains. However, correlations were small. The total score of the GAD-7 did not significantly correlate with any neurocognitive measure.
Table 4. Zero-order correlations and the regression coefficients (adjusted for age, sex, and education) between neurocognitive functioning, depression, and anxiety

a Transformed values were used in the analysis because test scores were not normally distributed.
Note. Higher scores on neuropsychological tests indicate better performance, except for divided attention. Correlations and regression coefficients were obtained using list-wise deletion. Significant correlations and regression coefficients are printed in bold.
When adjusting for sex, age, and education, the total score of the PHQ-9 was significantly associated with sustained attention (β = −0.13, p = 0.044), information processing speed (β = −0.20, p = 0.002), working memory (β = −0.17, p = 0.016), verbal memory (β = −0.14, p = 0.037), and phonological verbal fluency (β = −0.15, p = 0.036), suggesting that a higher depression score was associated with an impaired neurocognitive performance within these domains. The total score of the GAD-7 was significantly associated with lower information processing speed (β = −0.16, p = 0.018) and visual memory (β = −0.14, p = 0.044), indicating that a higher score of anxiety was associated with impaired neurocognitive performance within these domains.
Neurocognitive dysfunctioning of patients with SSRD and with comorbid depression and with comorbid anxiety
When examining presence v. absence of depression or anxiety, similar results were observed. MANOVA suggested that comorbid depression in patients with SSRD was associated with neurocognitive dysfunctioning (F(7, 161) = 2.489, p = 0.019, η 2 = 0.098), whereas anxiety in SSRD was not associated with neurocognitive dysfunctioning [F(7, 161) = 0.492, p = 0.839, η 2 = 0.021].
Table 3 (columns 3–8) displays the percentages of patients with a neurocognitive disorder, a neurocognitive deficit and patients without a neurocognitive disorder. For each neurocognitive domain, deficits are described for SSRD+D v. SSRD-D, and SSRD+A v. SSRD-A. In patients with SSRD+D, significantly more deficits (22.8%) and disorders (11.0%) were found within divided attention than in patients with SSRD-D (deficits/disorder, 7.3/4.9%, respectively). Fisher's exact tests yielded this difference significant (χ2 = 7.18, p = 0.027). Neurocognitive deficits/disorders (36.6% and 15.2%, respectively) were also significantly more found in patients with SSRD+D (χ2 = 8.58, p = 0.013) for the domain of information processing speed than in patients with SSRD-D (29.2% and 2.1%, respectively). Working memory was also significantly more impaired (deficits/disorders, 39.9% and 8.8%, respectively) in patients with SSRD+D (χ2 = 9.24, p = 0.009) than in patients with SSRD-D. Phonological verbal fluency was also significantly more impaired (30.4% deficits) in patients with SSRD+D (χ2 = 4.37, p = 0.037) than in patients with SSRD-D. Consistent with the analyses based on the continuous GAD-7 anxiety scores, no significant differences with regard to percentages of neurocognitive dysfunctioning were found between SSRD+A v. SSRD-A amongst all neurocognitive domains.
Since 66.7% of the patients suffered from comorbid depression and anxiety, we also described neurocognitive functioning stratified for a patient with comorbid depression, with comorbid anxiety and with comorbid depression and anxiety. Table 5 (columns 2–5) describe the percentages of patients with a neurocognitive disorder, a neurocognitive deficit and without a neurocognitive disorder for each neurocognitive domain stratified for comorbid depression and anxiety, comorbid depression, and comorbid anxiety.
Table 5. Neurocognitive functioning of the study sample (N = 201) of somatic symptom and related disorders and stratified for comorbid depression and anxiety, comorbid depression, comorbid anxiety, and no comorbid depression or anxiety

Note. Patients were assigned to the group with comorbid depression and anxiety if both scores of the PHQ-9 and GAD-7 were higher than the cut-off score.
Discussion
The present results suggest substantial impairments of information processing speed, sustained attention, divided attention, working memory, verbal memory, visual memory, and phonological verbal fluency in patients with SSRD. Within the domain of executive functioning (planning and mental flexibility), a relatively small percentage of impairments were found. Consistent with our hypotheses, a higher level of comorbid depression in patients with SSRD intensifies neurocognitive dysfunctioning, particularly impairments in the domains of divided attention, information processing speed, and working memory intensified. Contrary to our hypothesis, comorbid anxiety in SSRD was not significantly associated neurocognitive dysfunctioning.
Previous studies that focused on neurocognitive dysfunctioning of patients with somatoform disorder reported impaired executive functioning (Al-Adawi et al. Reference Al-Adawi, Al-Zakwani, Obeid and Zaidan2010; Demir et al. Reference Demir, Celikel, Taycan and Etikan2013; Brown et al. Reference Brown, Nicholson, Aybek, Kanaan and David2014). However, we found relatively low levels of impairment within the domain of executive functioning and documented more deficits in sustained attention, information processing speed, and working memory. One explanation for this discrepancy is that the neurocognitive profiles of patients with somatoform disorders do not fully overlap with the neurocognitive profile of patients with SSRD. Another possible explanation for this finding is that the subtests of the BADS are not sensitive enough to detect mild impairment in executive functioning (Chamberlain, Reference Chamberlain2003). This explanation seems plausible, because, in contrast to the current literature (Murrough et al. Reference Murrough, Iacoviello, Neumeister, Charney and Iosifescu2011; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Bennabi et al. Reference Bennabi, Vandel, Papaxanthis, Pozzo and Haffen2013; Snyder, Reference Snyder2013; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014) we found relatively low percentages of executive deficits associated with comorbid depression. Future studies should include tests that are more sensitive to mild impairment in executive functioning such as the Wisconsin Card Sorting Test (Heaton, Reference Heaton1981) and the Tower of London (Shallice, Reference Shallice1982).
In addition, executive functioning also includes a system of interconnected behaviors and thus consists of more components than planning and mental flexibility (Stuss & Benson, Reference Stuss and Benson1986; Fuster, Reference Fuster1997). Therefore, the absence of neurocognitive dysfunctioning within planning or mental flexibility does not necessarily indicate an absence of problems in the whole spectrum of executive functioning. In fact, we found substantial percentages of impairment in phonological verbal fluency, which is also part of executive functioning (Fisk & Sharp, Reference Fisk and Sharp2004). Because of these inconsistent results, conclusions about the executive functioning of patients with SSRD requires further investigation.
In addition to a previous study that already reported the presence of neurocognitive dysfunctioning in general (Inamura et al. Reference Inamura, Shinagawa, Nagata, Tagai, Nukariya and Nakayama2015), this study provides a detailed description of neurocognitive dysfunctioning of patients with SSRD. Our results show that patients with SSRD and depression experience more neurocognitive dysfunction than patients with SSRD without depression. Previous studies suggested that patients with severe depressive symptoms are more likely to experience memory difficulties than in patients with minimal to moderate depressive symptoms (Wang et al. Reference Wang, Halvorsen, Sundet, Steffensen, Holte and Waterloo2006; Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012). Our sample consisted of patients with SSRD and moderately severe depression (mean PHQ-9 score in total sample equal to 14.3), which may explain why we did not find enhanced memory problems in patients with SSRD+D. Memory problems are, in contrast to attentional and executive dysfunctioning, not a trait-marker for a major depressive disorder since memory deficits do not persist after remission of depressive symptoms (Lee et al. Reference Lee, Hermens, Porter and Redoblado-Hodge2012; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014). Therefore, memory problems in SSRD might be more dependent on the severity of depressive symptoms (state-marker) and will thus only present themselves in patients with severe depression. To explore whether or not memory problems are state dependent in patients with SSRD, examination of differences in memory functioning between patients with minimal to moderate depression (PHQ-9 < 15) and moderately severe to severe depression (PHQ-9 ⩾ 15) (Kroenke & Spitzer, Reference Kroenke and Spitzer2002) is warranted.
Our results did not support our hypothesis that anxiety affects neurocognitive dysfunctioning of patients with SSRD. However, previous studies reported impaired executive functioning, memory, attention, and learning for patients suffering from an anxiety disorder (De Geus et al. Reference De Geus, Denys, Sitskoorn and Westenberg2007; Castaneda et al. Reference Castaneda, Tuulio-Henriksson, Marttunen, Suvisaari and Lonnqvist2008; Harkin & Kessler, Reference Harkin and Kessler2011; Polak et al. Reference Polak, Witteveen, Reitsma and Olff2012; Tempesta et al. Reference Tempesta, Mazza, Iaria, De Gennaro and Ferrara2012; Tempesta et al. Reference Tempesta, Mazza, Serroni, Moschetta, Di Giannantonio, Ferrara and De Berardis2013), but none of these studies focused on the influence of comorbid anxiety on neurocognitive dysfunctioning of patients with SSRD. The present results suggest that depression, rather than anxiety intensifies neurocognitive dysfunctioning on several domains in SSRD patients. However, to explore the role of severe anxiety on neurocognitive dysfunctioning of patients with SSRD, examination of patients with severe anxiety (GAD-7 > 15) (Löwe et al. Reference Löwe, Decker, Müller, Brähler, Schellberg, Herzog and Herzberg2008) is warranted.
To our knowledge, this is the first study that investigated associations of neurocognitive dysfunctioning with depression and anxiety in patients with SSRD. In addition, this study excluded patients who were suspected of malingering which prevents that the results are biased by invalid conclusions regarding neurocognitive dysfunctioning of patients with SSRD in our sample. Even though our exclusion criteria included an IQ estimated above 80, seven patients were included with an IQ below 80 and 10 patients had an IQ within the range of 80–87 (corresponding to 2.4–20.0%). This may have influenced the result so caution should be exercised while interpreting the results. However, sensitivity analysis showed that patients with an IQ below 80 were significantly older but did not differ with regard to any other demographic characteristic and regarding mean PHQ-9 and GAD-7 scores. We therefore decided to include them in the further analyses of this study. Furthermore, our sample was composed of 62% women so the question whether or not gender influences the association of depression withneurocognitive functioning rather than depression alone arises. However, analyses showed that within women, depression was not significantly associated with impaired neurocognitive functioning so we considered gender not a factor of influence.
Implications of the present study require an evaluation of several methodological limitations. Selection bias might have occurred since patients at CLGG have to fit certain selection criteria to be eligible for clinical evaluation. Therefore, our results should be interpreted cautiously regarding generalization to groups with less severe SSRD. Furthermore, the symptom validity task was not administered to all patients because of the limited availability of the symptom validity tests (i.e., TOMM and AKTG). In the case of two or more simultaneous intakes, some patients could not be tested with a symptom validity task. As a consequence, so some patients might have scored positive on malingering but were included in this study. However, since only 12 of the 165 patients were suspected of malingering, we estimate the number of patients who are suspected of malingering in the non-administered group to be relatively small and their impact on the results to be minor. Moreover, other factors might have influenced neurocognitive dysfunctioning and were not taken into accounts, such as medication use and other comorbidities (e.g., attention deficit-hyperactivity disorder) (Alderson et al. Reference Alderson, Kasper, Hudec and Patros2013; Mowinckel et al. Reference Mowinckel, Pedersen, Eilertsen and Biele2015). It is also possible that the joint presence of depression and anxiety may have had disproportionate adverse effects on neurocognitive dysfunctioning of patients with SSRD. We described neurocognitive dysfunctioning of patients with comorbid depression and anxiety. However, these results should be interpreted cautiously because our sample included very few patients with comorbid anxiety which prevents us to draw solid conclusions whether or not comorbid depression and anxiety intensifies neurocognitive dysfunctioning compared with comorbid depression or comorbid anxiety in patients with SSRD. To conclude, a relationship between severity of SSRD and severity of depressive symptoms as related to neurocognitive functioning may be present and may explain our results. Future studies are needed to explore whether or not the severity of depression and severity of SSRD independently influence ttthe neurocognitive functioning of patients with SSRD.
CBT is the most frequently used therapy for treating the psychological disorder in SSRD patients (Kroenke, Reference Kroenke2007) but the effectivity of this treatment may be influenced negatively by neurocognitive dysfunctioning (i.e., patients may forget to do homework or homework assignments may be too demanding). A recent case description describes the negative effect of severe neurocognitive impairment within information processing speed on CBT, in a patient with conversion disorder. CBT had to be paused and the patient was offered Cognitive Rehabilitation Treatment (CRT). After CRT, neurocognitive functioning improved and CBT was successfully continued (de Vroege et al. Reference De Vroege, Khasho, Foruz and van der Feltz-Cornelis2017). Although this case report is the first to report successful influence on CBT via CRT in a patient with conversion disorder, this finding does suggest that patients with severe impairment (disorders within the neurocognitive of information processing speed) are less likely to be able to engage in CBT.
Conclusions
We conclude that neurocognitive dysfunctioning is present in the majority of patients with SSRD and that these impairments occur across different neurocognitive domains. Depression intensifies neurocognitive functioning mainly within the domains of sustained attention, information processing speed, working memory, verbal memory, and phonological verbal fluency. However, future studies with larger samples are needed to document the potential synergy between depression and anxiety and the influence on the neurocognitive functioning of patients with SSRD. This finding implies that a patient-centered personalized approach is warranted including awareness of neurocognitive dysfunctioning within SSRD. Furthermore, Future randomized controlled studies need to explore the effectivity of neurocognitive treatments with a repeated NPA to evaluate the improvement of the neurocognitive functioning of patients with SSRD.
Acknowledgements
We would like to thank Annick van Manen and Eva van der Thiel (psychologists) for their help obtaining the data from the NPA and patients files. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of Interest
None.








