Introduction
Developmental sex differences during normal development
From the prenatal period to adulthood, the human brain undergoes extensive development including progressive and regressive neuroanatomical changes (Giedd & Rapoport, Reference Giedd and Rapoport2010; Gilmore et al., Reference Gilmore, Shi, Woolson, Knickmeyer, Short, Lin and Shen2012; Gogtay et al., Reference Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis and Thompson2004; Raznahan et al., Reference Raznahan, Shaw, Lalonde, Stockman, Wallace, Greenstein and Giedd2011, Reference Raznahan, Shaw, Lerch, Clasen, Greenstein, Berman and Giedd2014). Sex-based differences have been documented in the volume of the hippocampus, cerebellum, thalamus, and the basal ganglia, as well as cortical thickness, demonstrating the impact of sex on neuroanatomical structures during normal development (Sowell et al., Reference Sowell, Peterson, Kan, Woods, Yoshii, Bansal and Toga2007; Sussman, Leung, Chakravarty, Lerch, & Taylor, Reference Sussman, Leung, Chakravarty, Lerch and Taylor2016). Accumulating evidence suggests that multiple factors contribute to the effects of sex differences on brain maturation (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, Reference Giedd, Castellanos, Rajapakse, Vaituzis and Rapoport1997; Gur & Gur, Reference Gur and Gur2017; Ruigrok et al., Reference Ruigrok, Salimi-Khorshidi, Lai, Baron-Cohen, Lombardo, Tait and Suckling2014), including genetic, environmental, cultural, and hormonal influences (Gogos, Ney, Seymour, Van Rheenen, & Felmingham, Reference Gogos, Ney, Seymour, Van Rheenen and Felmingham2019). Estradiol and progesterone contribute to sex differences in brain development according to their effects at receptors implicated in neurogenesis, microglial expression, inflammation, and bioenergetics (Gogos et al., Reference Gogos, Sbisa, Sun, Gibbons, Udawela and Dean2015; Rettberg, Yao, & Brinton, Reference Rettberg, Yao and Brinton2014; Sun, Walker, Dean, Van Den Buuse, & Gogos, Reference Sun, Walker, Dean, Van Den Buuse and Gogos2016) and contribute to the modulation and regulation of neurotransmitter activity within dopaminergic, serotonergic, glutamatergic, and GABAergic systems (Gogos et al., Reference Gogos, Sbisa, Sun, Gibbons, Udawela and Dean2015; Kokras et al., Reference Kokras, Pastromas, Papasava, De Bournonville, Cornil and Dalla2018; Sun et al., Reference Sun, Walker, Dean, Van Den Buuse and Gogos2016). Beyond the biological factors influencing brain development, culture and social environmental factors, such as distinct gender roles and cultural expectations toward boys and girls play an important role (Andermann, Reference Andermann2010).
Sex differences in neuropsychiatric conditions
The influence of sex on the brain is evident in neuropsychiatric conditions and neurodevelopmental disorders (Biederman et al., Reference Biederman, Mick, Faraone, Braaten, Doyle, Spencer and Johnson2002; Jacobs et al., Reference Jacobs, Ameis, Ji, Viviano, Dickie, Wheeler and Voineskos2019; Kaczkurkin, Raznahan, & Satterthwaite, Reference Kaczkurkin, Raznahan and Satterthwaite2019; May, Adesina, McGillivray, & Rinehart, Reference May, Adesina, McGillivray and Rinehart2019; Pinares-Garcia, Stratikopoulos, Zagato, Loke, & Lee, Reference Pinares-Garcia, Stratikopoulos, Zagato, Loke and Lee2018; Rutter, Caspi, & Moffitt, Reference Rutter, Caspi and Moffitt2003) with male preponderance in childhood-onset disorders and female preponderance in adolescent-onset disorders (Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020; Rutter et al., Reference Rutter, Caspi and Moffitt2003). Attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder, intellectual disability, and conduct disorder occur more frequently in boys than in girls (Biederman et al., Reference Biederman, Mick, Faraone, Braaten, Doyle, Spencer and Johnson2002; Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020; Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, Mcgrath and Eaton2014; Thapar & Cooper, Reference Thapar and Cooper2016); whereas, anxiety disorder, obsessive-compulsive disorder, mood disorder, and eating disorders are more commonly seen among girls, emerging in adolescence (Altemus, Sarvaiya, & Neill Epperson, Reference Altemus, Sarvaiya and Neill Epperson2014; Biederman et al., Reference Biederman, Mick, Faraone, Braaten, Doyle, Spencer and Johnson2002; Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020; Lewinsohn, Rohde, & Seeley, Reference Lewinsohn, Rohde and Seeley1998). Register studies show conflicting results, a study reported a higher incidence of schizophrenia amongst adolescent girls v. boys (Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020), whereas an earlier register-based study reported a similar incidence of schizophrenia in childhood and adolescence among both sexes (Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, Mcgrath and Eaton2014). In adulthood, men have a higher incidence of schizophrenia (Kühl, Laursen, Thorup, & Nordentoft, Reference Kühl, Laursen, Thorup and Nordentoft2016; Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, Mcgrath and Eaton2014; Thorup, Waltoft, Pedersen, Mortensen, & Nordentoft, Reference Thorup, Waltoft, Pedersen, Mortensen and Nordentoft2007) whereas after 50 years of age, women have a higher incidence of schizophrenia than men (Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, Mcgrath and Eaton2014; Thorup et al., Reference Thorup, Waltoft, Pedersen, Mortensen and Nordentoft2007). The cumulative incidence of bipolar disorder is higher for girls in childhood and adolescence than for boys (Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020) and higher for women compared with men (Pedersen et al., Reference Pedersen, Mors, Bertelsen, Waltoft, Agerbo, Mcgrath and Eaton2014).
Although individuals who develop schizophrenia in adulthood may exhibit cognitive, social, and motor problems in childhood (Cannon et al., Reference Cannon, Caspi, Moffitt, Harrington, Taylor, Murray and Poulton2002; Howes & Murray, Reference Howes and Murray2014; Murray, Bhavsar, Tripoli, & Howes, Reference Murray, Bhavsar, Tripoli and Howes2017; Niemi, Suvisaari, Tuulio-Henriksson, & Lonnqvist, Reference Niemi, Suvisaari, Tuulio-Henriksson and Lonnqvist2003; Walker, Savoie, & Davis, Reference Walker, Savoie and Davis1994), few studies have examined whether these developmental problems differ according to sex (Marcus, Reference Marcus1985a; Weiser et al., Reference Weiser, Reichenberg, Rabinowitz, Kaplan, Mark, Nahon and Davidson2000). Similarly, although children of parents with bipolar disorder were found to display sleep difficulties and anxiety during childhood (Duffy et al., Reference Duffy, Horrocks, Doucette, Keown-Stoneman, Mccloskey and Grof2014), sex differences were not examined. However, one of the risk factors, specifically for boys when developing bipolar disorder was excellent school performance (Maccabe et al., Reference Maccabe, Lambe, Cnattingius, Sham, David, Reichenberg and Hultman2010).
Because of the neuroanatomical, genetic, hormonal, and cultural effects of sex during brain development and the influence of sex differences in neuropsychiatric conditions, establishing whether sex differences manifest early in development in at-risk children could facilitate early detection.
Aims of the study
The overarching objective of the Danish High Risk and Resilience Study was to assess the influences of familial risk and environmental factors in children with a familial high risk of schizophrenia (FHR-SZ) or bipolar disorder (FHR-BP). Furthermore, to identify early risk markers of schizophrenia and bipolar disorder to establish a basis for future primary preventive interventions in the premorbid phase. The purpose of this sub-study was to investigate whether groups of boys and girls with a FHR-SZ, FHR-BP, and controls differed in terms of neurocognition, language, motor function, psychopathology, social cognition, social behavior, home environment, and global functioning before puberty. Moreover, we assessed potential sex differences in symptomatology and function amongst children with FHR-SZ, FHR-BP, and controls. Given the existing sex-based differences in the incidence of schizophrenia, we hypothesized that we would find sex-based differences between the familial high-risk groups (sex-by-group interaction), and specifically, that we would observe poorer function and outcome in boys in the FHR-SZ group compared with girls in the FHR-SZ group and control boys. Because females have a higher cumulative incidence than males of bipolar disorder, we hypothesized that we would observe poorer function and outcome in FHR-BP girls compared with FHR-BP boys and control girls.
Methods and materials
The Danish Data Protection Agency approved the study protocol. We obtained authorization to draw data from registers from the Danish Ministry of Health. The Danish National Committee on Health Research Ethics received the protocol. Because of the noninterventional study design, ethical approval was not considered necessary by the authority. The legal guardians of the participating children provided written informed consent.
Study design and participants
The Danish High Risk and Resilience Study-VIA7 took place in Denmark between 1 January 2013 and 31 January 2016 (Thorup et al., Reference Thorup, Jepsen, Ellersgaard, Burton, Christiani, Hemager and Nordentoft2015). This stratified cohort consisted of 522 Danish children aged 7 years with either one parent, two parents, or neither parent diagnosed with schizophrenia spectrum disorder or bipolar disorder. We chose to assess the children at age 7 because in Denmark most children have started school at age 7. Beginning school denotes an important developmental step for a child, implying increased demands, cognitive and academic, as well as socially. We identified the cohort using The Danish Civil Registration System (DCRS) (Pedersen, Gotzsche, Moller, & Mortensen, Reference Pedersen, Gotzsche, Moller and Mortensen2006) and The Danish Psychiatric Central Research Register (DPCR) (Mors, Perto, & Mortensen, Reference Mors, Perto and Mortensen2011). Families were contacted to participate in the study by letter and subsequently by telephone (Fig. 1). Schizophrenia spectrum disorder was defined as schizophrenia, delusional disorder, or schizoaffective disorder, and was reflected by International Classification of Disease (ICD) 10-codes (F20, F22, and F25) or ICD 8-codes (295, 297, 298.29, 298.39, 298.89, and 298.99). Bipolar disorder was associated with ICD 10-codes (F30, F31) and ICD 8-codes (296.19 and 296.39). Controls were defined as population-based control children of parents with no diagnoses of schizophrenia spectrum disorders or bipolar disorder. The control children were matched to the FHR-SZ children according to sex, age, and municipality. The FHR-BP children were included as a non-matched group that was comparable to the other groups in terms of age and sex. The children underwent a battery of tests to assess neurocognition (Hemager et al., Reference Hemager, Plessen, Thorup, Christiani, Ellersgaard, Spang and Jepsen2018), social cognition and language (Christiani et al., Reference Christiani, Jepsen, Thorup, Hemager, Ellersgaard, Spang and Nordentoft2019), motor function (Burton et al., Reference Burton, Thorup, Jepsen, Poulsen, Ellersgaard, Spang and Plessen2017), psychopathology (Ellersgaard et al., Reference Ellersgaard, Jessica Plessen, Richardt Jepsen, Soeborg Spang, Hemager, Klee Burton and Elgaard Thorup2018), and the home environment (Gantriis et al., Reference Gantriis, Thorup, Harder, Greve, Henriksen, Zahle and Bliksted2019), with group differences described elsewhere.

Fig. 1. Recruitment of participating children in The Danish High Risk and Resilience Study. aDanish National Registries: Danish Civil Registration System and Danish Psychiatric Central Research Register. bFHR-SZ: Children of parents with schizophrenia spectrum disorders. cDouble diagnosed parents: Parents with diagnoses of schizophrenia and bipolar disorder were assigned to the schizophrenia high-risk group as per the ICD-10 hierarchy. dFHR-BP: Children of parents with bipolar disorder. eControls: Population-based control children of parents with no diagnoses of schizophrenia spectrum disorders or bipolar disorder. fResearch protection: As of May 2011, legislation was enacted to protect individuals phone numbers from being called for participation in scientific research. Therefore, there were eligible children who were not contacted and enrolled in VIA 7. gControls selection: Up to 10 controls were retrieved for each child in the schizophrenia spectrum disorder group and the bipolar disorder group. Controls were matched to cases on sex, municipality and exact age. Control cases were matched to children in the schizophrenia familial high-risk group. hDefinition of contact: First through letters sent to the child´s address. If the family did not respond, contact by telephone was attempted (calls and text messages), if a phone number could be found. iRe-assigned control parent: One control parent was found to have a diagnosis of bipolar disorder made by a private doctor, therefore the diagnosis was not present/visible in the National Registry extract, as private doctors do not report to the National Registry. This family/parent was therefore reassigned to the bipolar disorder familial high-risk group. Therefore, the N = 201 for controls is now N = 200. jControl children not in the extract: Two younger siblings were included to VIA 7 by request of the parents. They were not in the original extract.
Outcome and procedure
The instruments selected were validated and specifically developed and selected for the age group, sensitive to small changes and suitable for later follow-up. Domain characteristics and outcomes are illustrated in Table 1.
Table 1. Characteristics and performance of 7- year old children with familial risk of schizophrenia, bipolar disorder or controls presented by sex and familial risk status within specified domains of function

FHR-SZ: children with familial risk of schizophrenia, FHR-BP: children with familial risk of bipolar disorder, controls: Population-based controls. Index parents refer to the biological parents with a diagnosis of schizophrenia spectrum psychosis or bipolar disorder. Higher scores in the social responsiveness domain indicate more problematic social behavior. RIST: The Reynold's Intellectual Screening test. Movement ABC-2: Movement Assessment Battery for Children – second edition. mADHD-RS: A modified version of the Attention Deficit Hyperactivity Disorder Rating Scale. RS K-SADS-PL: Schedule for Affective Disorders and Schizophrenia for School-Age Children- present and Lifetime Version.
a The primary caregiver to the child, is the legal guardian who spent the most time with the child. The primary caregiver completed CBCL and Vineland-II.
b Includes Trail-Making Test Number Sequencing, Trail-Making Test Letter Sequencing, Trail-Making Test Letter-Number Switching, Symbol Search, Coding, Arithmetic, Letter-Number Sequencing, and Spatial Working Memory; total errors.
c Includes Memory for Stories immediate recall, Memory for Stories delayed recall, Guess What, Verbal Fluency phonemic, Verbal Fluency semantic, and Verbal Fluency switching.
d Includes Stockings of Cambridge Problems Solved in Minimum Moves, Spatial Span length, Odd-Item Out, Intra-Extra Dimensional Set Shift extra-dimensional stage errors, and Spatial Recognition Memory percentage correct.
e Includes Rey Complex Figure Test and Recognition Recall, Word Selective Reminding Immediate Recall, Word Selective Reminding Delayed Recall, and Rapid Visual Information Processing.
Neurocognition
Reynold's Intellectual Screening test (Reynolds & Kamphaus, Reference Reynolds and Kamphaus2003) was used to measure general intelligence. Neurocognitive function was assessed using subtests from the Wechsler Intelligence Scale for Children – fourth edition (Wechsler, Reference Wechsler2003), Delis-Kaplan Executive Function System (Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001), Test of Memory and Learning – Second Edition (Reynolds & Voress, Reference Reynolds and Voress2007), Cambridge Neuropsychological Test Automated Battery (Sahakian & Owen, Reference Sahakian and Owen1992), and Rey Complex Figure Test and Recognition Trial (Meyers & Meyers, Reference Meyers and Meyers1995) and scores were converted to z-scores based on the control group with both sexes included. To reduce the number of variables, we derived four neurocognitive components (Processing speed and working memory, Verbal function, Executive and visuospatial functions, and Declarative memory and attention) after conducting principal component analysis [described elsewhere (Hemager et al., Reference Hemager, Plessen, Thorup, Christiani, Ellersgaard, Spang and Jepsen2018)].
Language
Receptive language was evaluated using the Test for Reception of Grammar-2 (Bishop, Reference Bishop2010).
Motor
Motor function was assessed using the Movement Assessment Battery for Children – second edition (Henderson, Sugden, & Barnett, Reference Henderson, Sugden and Barnett2007). Raw scores were converted to standard scores using the normative data from the Movement ABC-2 manual, as described elsewhere (Burton et al., Reference Burton, Thorup, Jepsen, Poulsen, Ellersgaard, Spang and Plessen2017).
Psychiatric diagnosis
The psychiatric diagnoses were established using the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL) (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997).
Psychopathology and functioning
A modified version of the ADHD Rating Scale (Barkley, Gwenyth, & Arthur, Reference Barkley, Gwenyth and Arthur1999; Dupaul, Power, & Anastopoulos, Reference Dupaul, Power and Anastopoulos1998; Makransky & Bilenberg, Reference Makransky and Bilenberg2014) was used to assess symptoms of ADHD and oppositional defiant disorder. These were completed by the teacher of each child. The Child Behavior Checklist (Achenbach & Rescorla, Reference Achenbach and Rescorla2001) was used to assess the severity of various dimensions of psychopathology; this was completed by the caregiver of each child (defined as the parent/legal guardian who spent the most time with the child). Each child's teacher filled out the Teacher's Report Form (Achenbach & Rescorla, Reference Achenbach and Rescorla2001). An interviewer evaluated the current level of functioning of each child using the Children's Global Assessment Scale (Shaffer et al., Reference Shaffer, Gould, Brasic, Ambrosini, Fisher, Bird and Aluwahlia1983), as part of the K-SADS-PL interview. The Test Observation Form, which assesses behavioral and emotional problems observed during an assessment session (Mcconaughy & Achenbach, Reference Mcconaughy and Achenbach2004), was also completed. Psychiatric diagnoses and psychopathology are described elsewhere (Ellersgaard et al., Reference Ellersgaard, Jessica Plessen, Richardt Jepsen, Soeborg Spang, Hemager, Klee Burton and Elgaard Thorup2018).
Social cognition
Theory of mind was assessed using Strange Stories-Revised (White, Hill, Happé, & Frith, Reference White, Hill, Happé and Frith2009), and facial affect recognition was assessed via the computerized emotion recognition task from the Cambridge Automated Neuropsychological Test Battery (Sahakian & Owen, Reference Sahakian and Owen1992).
Social behavior
Social behavior was conceptualized as social responsiveness and adaptive social functioning, as assessed by the child's teacher via the Social Responsiveness Scale (Constantino et al., Reference Constantino, Davis, Todd, Schindler, Gross, Brophy and Reich2003). The primary caregiver completed the Vineland-II Socialization subdomain (Sparrow, Cincchetti, & Balla, Reference Sparrow, Cincchetti and Balla2006). The tests of social cognition, social behavior, and language are described elsewhere (Christiani et al., Reference Christiani, Jepsen, Thorup, Hemager, Ellersgaard, Spang and Nordentoft2019).
Home environment
The quality of the home environment was assessed with the Middle Childhood-HOME Inventory (Caldwell & Bradley, Reference Caldwell and Bradley2003) evaluating the level of stimulation and support in the home, which is described elsewhere (Gantriis et al., Reference Gantriis, Thorup, Harder, Greve, Henriksen, Zahle and Bliksted2019). Sex differences among children living with an index parent (defined as a parent with a diagnosis of either schizophrenia spectrum disorder or bipolar disorder, or a matched control parent) were assessed.
Statistical analyses
Summary statistics for demographic, clinical and domain characteristics were calculated by sex and familial risk group (FHR-SZ, FHR-BP or control). Differences in mean values were analyzed bivariately by means of an analysis of variance for continuous outcomes and by chi-square test for categorical outcomes (Table 1). All variables listed in Table 1 were used for statistical analyses separately and all the outcomes from Table 1 are illustrated in Figs 2 and 3. We tested the influences of sex and familial-risk status as well as the effect modification of sex and familial-risk group on each of the continuous dependent variables in each domain (language, neurocognition, social cognition, social behavior, motor function, psychopathology and general functioning). This was done by fitting regression models including main effects of sex and familial-risk group and their interaction followed by a hierarchical F-test. All continuous outcomes were standardized to z-scores. For binary outcomes, logistic regression models were fitted using the same dependent variables to the following domains: psychiatric diagnosis (present/absent), home environment (having an insufficient home environment was defined as an MC-HOME Inventory total score ≤40) (Gantriis et al., Reference Gantriis, Thorup, Harder, Greve, Henriksen, Zahle and Bliksted2019) and (living with index parent or not), regression on sex, group and the interaction sex-by-familial-risk-group. For all outcomes, pairwise comparisons between sexes and group were performed post hoc and visualized graphically (Figs 2 and 3 and Table 2). For continuous outcomes the pairwise comparison is the difference in Cohen's delta, where d = 0.2 is considered a ‘small’ effect size, 0.5 represents a ‘medium’ effect size and 0.8 a ‘large’ effect size (Cohen, Reference Cohen1988), while for binary outcomes the difference is in proportions. We applied a hierarchical testing principle to reduce the risk of type I error. First, for overall testing, the significance was set at 0.05. For all pairwise comparisons, a Bonferroni correction significance level of 0.001 was applied (i.e. 0.05/50 tests). Because of the small number of sibling pairs (n = 16 of which 6 pairs were twins), we did not consider the effect of a sibling or high genetic loading (nine children had two parents with schizophrenia or bipolar disorder) in the statistical model. All statistical analyses were conducted using R version 3.5.1 (2018-07-02).
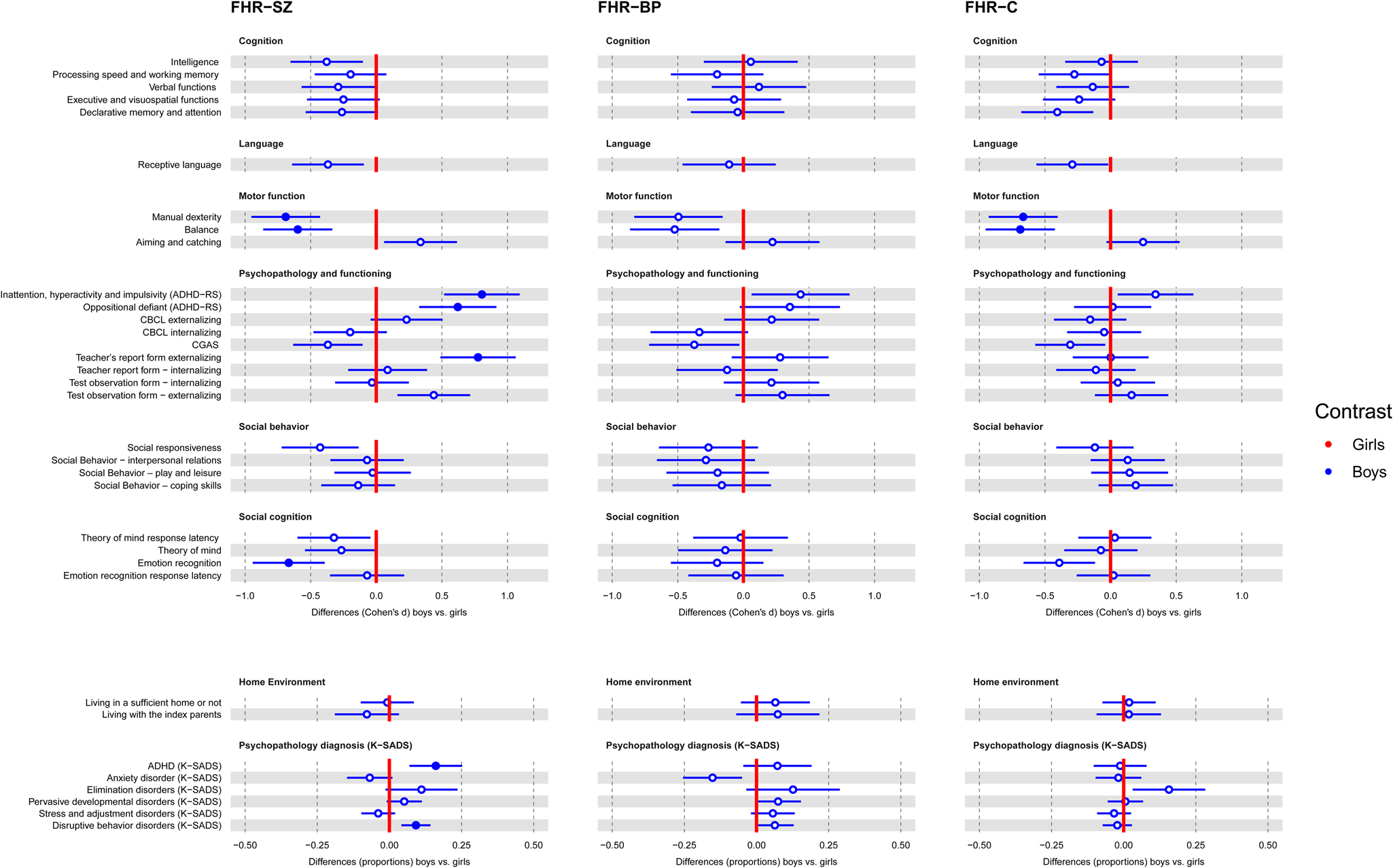
Fig. 2. Sex differences within familial-risk groups; FHR-SZ boys compared with FHR-SZ girls, FHR-BP boys compared with FHR-BP girls and control boys compared with control girls. Boys are illustrated in blue and girls in red as the contrast group. Differences are illustrated by effect size Cohen's d. For the domains of the home environment and psychopathology diagnoses differences are illustrated in proportions. Error bars indicate 95% confidence interval. Marked dots represent significant Bonferroni correction (p = 0.001). Estimates with clear dots and 95% confidence intervals which do not cross the red vertical contrast line represent a significance level of 5%. The direction of performance (worse/better) follows the instrument.
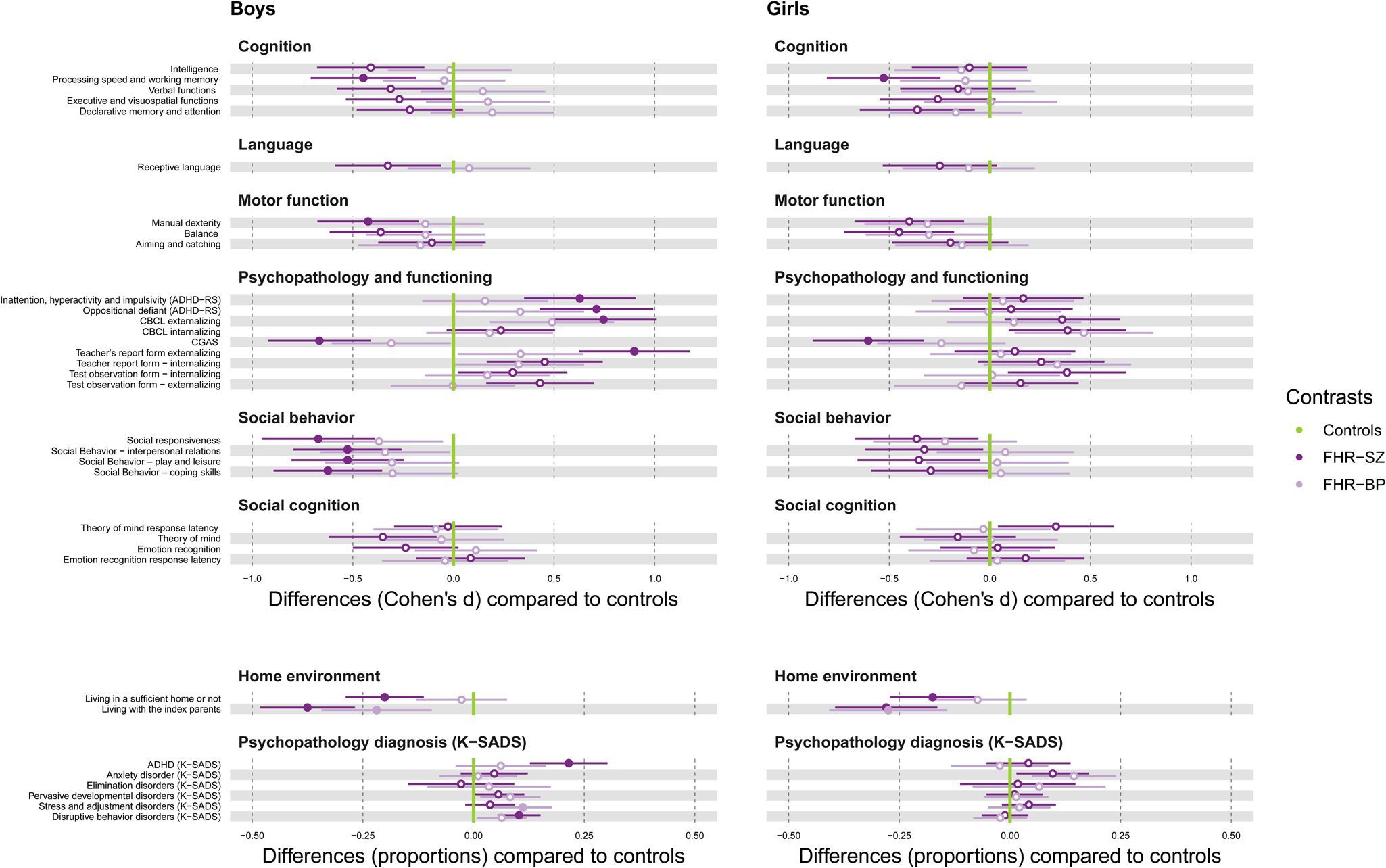
Fig. 3. Sex differences between familial-risk groups compared with controls. On the left FHR-SZ boys v. control boys and FHR-BP boys v. control boys. On the right FHR-SZ girls v. control girls and FHR-BP girls v. control girls. FHR-SZ are illustrated in dark purple, FHR-BP in light purple and controls in the green vertical line. Differences are illustrated by effect size Cohen's d. For the domains of the home environment and psychopathology diagnoses differences are illustrated in proportions. Error bars indicate 95% confidence interval. Marked dots represent significant Bonferroni correction (p = 0.001). Estimates with clear dots and 95% confidence intervals which do not cross the green vertical control line represent a significance level of 5%. The direction of performance (worse/better) follows the instrument.
Table 2. Pairwise comparisons between sex and familial high-risk status
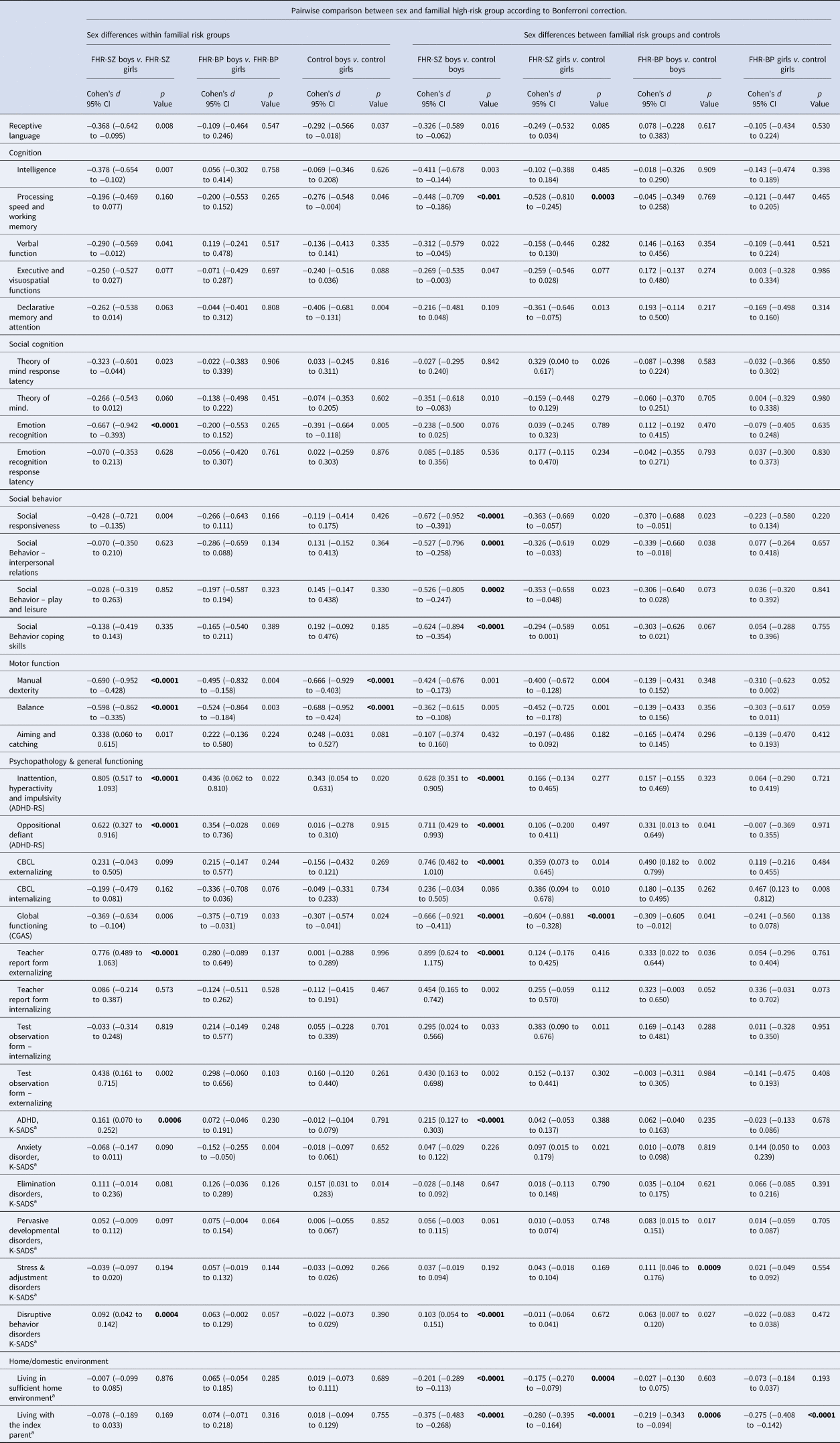
The pairwise comparison is the difference in Cohen's delta, where d = 0.2 is considered a ‘small’ effect size, 0.5 represents a ‘medium’ effect size and 0.8 a ‘large’ effect size while for binary outcomes (marked as a) the difference is in proportions. Due to the multiple comparisons we used Bonferroni correction for the post-hoc pairwise comparisons and considered p values <0.001 (0.05/50 tests) as significant (marked in bold), to reduce the risk of type I errors when performing multiple comparisons. Index parents refer to the biological parents with either diagnosis of schizophrenia spectrum psychosis or bipolar disorder and their adult matched control.
Results
The VIA-7 cohort included 522 children aged 7 years; 202 (39%) with FHR-SZ [93 girls (46%)], 120 (23%) with FHR-BP [56 girls (46.7%)] and 200 (38%) controls [93 girls (46.5%)]. No significant difference between sexes (p = 0.99) nor age at inclusion (p = 0.09) were observed across all groups. Demographic characteristics and the various domains are reported in Table 1.
Sex differences modified by familial-risk groups
Significant effect modifications (sex had different implications in different groups) were seen for four domains: oppositional defiant (ADHD-RS), teacher report form externalizing, disruptive behavior disorders (K-SADS) and stress & adjustment disorders (K-SADS). The significant interaction between group and sex for the domain of oppositional defiant (ADHD-RS), rated by the teacher (F = 4.11, df = 2, p = 0.017) denoted that the sex difference was greater in the FHR-SZ group compared with the sex difference in the other groups with a medium effect (Cohen's d = 0.622, p < 0.0001). Furthermore, the effect modification between group and sex for teacher-rated externalizing behavior (F = 7.13, df = 2, p < 0.001) signified the sex difference was greater in the FHR-SZ group compared with the sex difference in the other groups with a medium effect (Cohen's d = 0.776, p < 0.0001) (Table 2).
The significant effect modification between group and sex for the domains of K-SADS diagnosis disruptive disorders (Residual dev. = 130.8, df = 2, p = 0.008) revealed the sex difference was greater in the FHR-SZ group compared with the other groups; denoting the FHR-SZ boys had 9.19% higher proportion of disruptive behavior disorders, (p = 0.0004) compared with FHR-SZ girls (Fig. 2), whereas no sex difference were evident within the FHR-BP (p = 0.057) or the control group (p = 0.39) (Table 2). For stress and adjustment disorders (Residual dev = 177.2, df = 2, p = 0.033), the sex difference was larger in the FHR-BP group compared with controls. FHR-BP boys had an 11.11% higher proportion of stress and adjustment disorder compared with control boys (p = 0.0009), whereas there was no difference between FHR-BP girls compared with control girls (p = 0.55) (Fig. 3). For the remaining domains (described in Table 2, Figs 2 and 3) no significant modifications between sex and group were evident.
Sex differences within the familial-risk group
Sex differences within familial risk groups is shown in Fig. 2 and Table 2. Overall, sex had a significant impact on various domains among FHR-SZ children, whereas sex did not affect the tested domains in FHR-BP children (Fig. 2).
FHR-SZ boys v. FHR-SZ girls
Figure 2. FHR-SZ boys compared with FHR-SZ girls exhibited poorer social cognition in relation to emotion recognition with a medium effect (Cohen's d = −0.667, p < 0.0001), poorer motor skills with regard to manual dexterity (Cohen's d = −0.690, p < 0.0001) and balance with a medium effect (Cohen's d = −0.598, p < 0.0001). FHR-SZ boys had a 16.08% higher proportion of ADHD diagnoses (p = 0.0006) and a 9.19% higher proportion of disruptive behavior disorders (p = 0.0004) compared with FHR-SZ girls. The teachers of the children rated higher levels of inattention, hyperactivity and impulsivity with a large effect (Cohen's d = 0.805, p < 0.0001). We detected no differences in the home environment between boys or girls having a parent diagnosed with schizophrenia (difference in proportion = −0.007, p = 0.876). Furthermore, we found no sex differences between FHR-SZ boys and FHR-SZ girls in the domains of cognition, language, social behavior and home environment (FHR-SZ in Fig. 2) and Table 2.
FHR-BP boys v. FHR-BP girls
We detected no significant effects of sex after Bonferroni corrections between boys of parents with bipolar disorder compared with girls of parents with bipolar disorder, (FHR-BP in Fig. 2).
Control boys v. control girls
We found sex-based differences in motor function among the control children after Bonferroni correction. Boys displayed poorer manual dexterity (Cohen's d = −0.666, p < 0.0001) and balance (Cohen's d = −0.688, p < 0.0001) both with a medium effect compared with the girls from the control group.
Sex differences between familial-risk groups and controls
FHR-SZ boys compared with control boys
Figure 3. FHR-SZ boys showed impaired processing speed and working memory with a medium effect (Cohen's d = −0.448, p < 0.001), and poorer manual dexterity with a small to medium effect (Cohen's d = −0424, p < 0.001) compared with control boys (Boys in Fig. 3). FHR-SZ boys had a higher proportion (21.5%) of ADHD diagnoses (p < 0.0001) and a higher proportion (10.28%) of disruptive behavior disorders (p < 0.0001) compared with boys from the control group. Teachers reported a higher degree of externalizing behavior problems among FHR-SZ boys (Cohen's d = 0.8995, p < 0.0001) compared with control boys with a large effect. Teachers reported higher levels of inattention, hyperactivity and impulsivity symptoms in FHR-SZ boys (Cohen's d = 0.628, p < 0.0001), as well as oppositional defiant behavior (Cohen's d = 0.711, p < 0.0001) compared with control boys with a medium effect. FHR-SZ boys showed less social responsiveness (Cohen's d = −0.672, p < 0.0001) and poorer adaptive social functioning: interpersonal relations (Cohen's d = −0527, p = 0.0001), play and leisure (Cohen's d = −0.526, p = 0.0002) and coping skills (Cohen's d = −0.624, p < 0.0001) with a medium effect compared with control boys. FHR-SZ boys demonstrated lower global functioning than the control boys with a medium effect (Cohen's d = −0.666, p < 0.0001). Furthermore, 20.07% of FHR-SZ boys lived in insufficient home environments compared with boys from the control group (p < 0.0001). A higher proportion (37.53%) of FHR-SZ boys did not live with the index parent (i.e. the parent diagnosed with schizophrenia spectrum disorder) compared with boys from the control group who lived with the control index parent (the parent that was matched to the index parent) (p < 0.0001), Fig. 3. No group differences were detected after Bonferroni correction between FHR-SZ boys compared with control boys in the domain of language and social cognition.
FHR-SZ girls compared with control girls
FHR-SZ girls showed impaired processing speed and working memory (Cohen's d = −0.528, p = 0.0003), and lower global functioning (Cohen's d = −0.604, p < 0.0001) compared with control girls with a medium effect. A higher proportion (27.96%) of FHR-SZ girls did not live with the index parent compared with control girls who lived with the control index parent (p < 0.0001). A higher proportion (17.46%) of FHR-SZ girls lived in an inadequate home environment compared with control girls (p = 0.0004), (girls in Fig. 3). No group differences were detected between FHR-SZ girls compared with control girls in the domains of language, motor function, social behavior, social cognition and psychopathology.
FHR-BP boys or girls compared with control boys or girls respectively
A higher proportion of both FHR-BP boys (21.89%), (p = 0.0006) and FHR-BP girls (27.48%), (p < 0.0001) did not live with their parent diagnosed with bipolar disorder compared with control boys or girls respectably. For all other domains, no group differences were detected for FHR-BP boys or girls (Fig. 3).
Discussion
The findings from our register-based cohort of 522 7-year-old children showed for disruptive behavior (either assessed by semi-structural interview, by rating-scales filled out by the primary caregiver and teacher) that the sex difference was greater between boys and girls in the FHR-SZ group compared with the other groups; denoting the FHR-SZ boys had a higher proportion (or with a medium effect) of disruptive behavior compared with FHR-SZ girls. The sex difference for stress & adjustment disorders was larger for boys in the FHR-BP group compared with control boys, whereas no difference detected between FHR-BP girls v. control girls. No other modifications between sex and group were evident however, there were similar but large sex differences in the following domains: FHR-SZ boys performed poorer than FHR-SZ girls in manual dexterity and balance, emotion recognition, and had a higher proportion of ADHD diagnoses. We found no sex differences between FHR-BP boys and girls. Within the control group, boys showed poorer manual dexterity and balance compared with girls.
Compared with boys from the control group, FHR-SZ boys exhibited poorer processing speed and working memory, manual dexterity, and social behavior. Additionally, compared with control boys, FHR-SZ boys had a higher proportion of ADHD diagnoses and disruptive behavior disorder, as well as lower levels of general functioning. In addition, a higher proportion of FHR-SZ boys lived in inadequate homes compared with boys from the control group. Thus, impairments within neurodevelopmental domains were most frequently associated with FHR-SZ among boys.
Previous studies examining sex differences in children with a familial risk of schizophrenia or premorbid schizophrenia characteristics have shown that boys display more neurocognitive (Aylward, Walker, & Bettes, Reference Aylward, Walker and Bettes1984; Hans, Reference Hans1999), motor (Hans, Reference Hans1999; Marcus, Reference Marcus1985b), and behavioral (Watt & Lubensky, Reference Watt and Lubensky1976) impairments than girls.
Sex differences in motor abilities
Our motor ability findings, which reflect coordination skills, demonstrated sex-based differences both within the FHR-SZ group and within the control group, where boys performed worse than girls in manual dexterity and balance, signifying a possible stereotype sex pattern of motor function. Furthermore, we found that FHR-SZ boys exhibited poorer manual dexterity than control boys. Our results are consistent with evidence from previous studies of high-risk populations. The Copenhagen High-Risk Study, which assessed children of parents with schizophrenia, reported that Danish boys exhibited poorer performance than girls in terms of motor coordination and motor overflow at age 11–13 year (Marcus, Reference Marcus1985b). When the children from the Copenhagen High-Risk Study were assessed again at age 31–33 years, the offspring who developed schizophrenia exhibited significantly poorer premorbid coordination skills compared with those who did not develop a mental illness (Schiffman et al., Reference Schiffman, Sorensen, Maeda, Mortensen, Victoroff, Hayashi and Mednick2009). The Northern Finland Birth Cohort reported that boys who learned to stand without support after 12 months of age had a significantly higher risk of developing schizophrenia, and this was not the case for girls. The earlier the child stood unsupported, the lower the subsequent risk of schizophrenia (Isohanni et al., Reference Isohanni, Jones, Moilanen, Rantakallio, Veijola, Oja and Jarvelin2001). Furthermore, the Israeli High-Risk Study assessing 7–14 years old children of a parent with schizophrenia, showed that boys compared with girls exhibited impaired motor coordination and motor overflow/associated movement, but the effect of sex was no longer evident 5 years later (Marcus, Reference Marcus1985a). Future studies may evaluate whether motor sex differences are early childhood manifestations, which are transitory and disappear in adolescent/adulthood or whether they persist.
Sex differences in cognition
A cohort study examining premorbid cognitive sex differences at age 16–18 years showed that global cognitive performance/general intelligence was poorer in girls v. boys before they developed schizophrenia (Weiser et al., Reference Weiser, Reichenberg, Rabinowitz, Kaplan, Mark, Nahon and Davidson2000), whereas a meta-analysis showed premorbid IQ deficits were more prevalent among males than females (Aylward et al., Reference Aylward, Walker and Bettes1984). When controlling for multiple comparisons in the present study, we found that FHR-SZ boys and girls at age 7 years exhibited poorer processing speed and working memory compared with controls, but there were no sex-based differences in general intelligence, declarative memory and attention, verbal function, or executive and visuospatial function. Although children of parents with schizophrenia have been found to exhibit deficits in neurocognitive functioning compared with controls (Agnew-Blais & Seidman, Reference Agnew-Blais and Seidman2013; Cornblatt, Obuchowski, Roberts, Pollack, & Erlenmeyer-Kimling, Reference Cornblatt, Obuchowski, Roberts, Pollack and Erlenmeyer-Kimling1999; Erlenmeyer-Kimling et al., Reference Erlenmeyer-Kimling, Rock, Roberts, Janal, Kestenbaum, Cornblatt and Gottesman2000; Hameed & Lewis, Reference Hameed and Lewis2016; Ozan et al., Reference Ozan, Deveci, Oral, Karahan, Oral, Aydin and Kirpinar2010), studies examining children and adolescents with a familial risk of bipolar disorder have produced contrasting results. For instance, these children were found to exhibit deficient (Diwadkar et al., Reference Diwadkar, Goradia, Hosanagar, Mermon, Montrose, Birmaher and Keshavan2011; Hemager et al., Reference Hemager, Vangkilde, Thorup, Christiani, Ellersgaard, Spang and Plessen2019; Klimes-Dougan, Ronsaville, Wiggs, & Martinez, Reference Klimes-Dougan, Ronsaville, Wiggs and Martinez2006) and non-deficient attention capacities (Burton et al., Reference Burton, Vangkilde, Petersen, Skovgaard, Jepsen, Hemager and Plessen2018; Goetz et al., Reference Goetz, Novak, Viktorinova, Ptacek, Mohaplova and Sebela2019). Furthermore, none of the mentioned studies reported sex-based differences. Indeed, the heterogeneity of study methods in relation to age and neurocognitive function hinders the comparison of our results with previous data.
Neuropsychiatric and behavioral sex differences
Compared with FHR-SZ girls and controls boys, the FHR-SZ boys in our study showed more behaviors associated with conduct disorder and externalizing behavior problems. This finding is consistent with a previous report that teacher ratings of social maladjustment were higher in 7-year-old children who developed schizophrenia in adulthood (Done, Crow, Johnstone, & Sacker, Reference Done, Crow, Johnstone and Sacker1994). Furthermore, teacher ratings of over-reactive social maladjustment behavior (anxiety regarding acceptance, hostility, inconsequential behavior) were higher in boys v. girls who developed schizophrenia in adulthood, as well as typically developing children (Done et al., Reference Done, Crow, Johnstone and Sacker1994). Furthermore, studies documenting incidence rates derived from register-based nationwide cohorts of children have reported that ADHD and conduct disorder are more common among boys than girls in the general population (Dalsgaard et al., Reference Dalsgaard, Thorsteinsson, Trabjerg, Schullehner, Plana-Ripoll, Brikell and Pedersen2020). This supports our finding that FHR-SZ boys had a significantly higher proportion of ADHD diagnoses and disruptive behavior disorders compared with FHR-SZ girls. Moreover, children of parents with severe mental illness are known to have a higher risk of any mental disorder (Rasic, Hajek, Alda, & Uher, Reference Rasic, Hajek, Alda and Uher2014). However, the sex-based differences for these disorders were not evident among our control group. This may be due to the relatively low incidence of mental disorders in the general population at this early age, as well as our relatively small control group (n = 200), which may have contributed to a lack of power to detect sex-based differences.
The genetic and environmental exposure
Considering the home environment, our study showed no sex differences within the FHR-SZ group (FHR-SZ boys v. FHR-SZ girls), or within the FHR-BP group (FHR-BP boys v. FHR-BP girls) or within the control group (Fig. 2). However, a group difference between FHR-SZ and controls was evident showing a higher proportion of boys or girls with FHR-SZ compared with control boys or control girls respectively, lived in insufficient homes (Fig. 3). This was not evident for FHR-BP children compared with controls (Fig. 3). Even though we have tried to avoid environmental confounding by having a matched control group, where we controlled for municipality, sex and age, we could not control for the within family exposure. It would be unethical and impossible to randomized children to be exposed to poor environmental conditions to investigate the interaction between environmental and genetic risk factors.
We know that despite the considerable genetic risk, environmental factors also contribute to the risk of developing severe mental disorders (Van, Kenis, & Rutten, Reference Van, Kenis and Rutten2010). These environmental exposures range from prenatal factors such as maternal intrauterine infections (Borglum et al., Reference Borglum, Demontis, Grove, Pallesen, Hollegaard, Pedersen and Mors2014; Brown, Reference Brown2006; Mednick, Machon, Huttunen & Bonett, Reference Mednick, Machon, Huttunen and Bonett1988; Mortensen et al., Reference Mortensen, Norgaard-Pedersen, Waltoft, Sorensen, Hougaard, Torrey and Yolken2007) preterm birth and obstetric complications (Byrne, Agerbo, Bennedsen, Eaton & Mortensen, Reference Byrne, Agerbo, Bennedsen, Eaton and Mortensen2007), neonatal vitamin D status (McGrath et al., Reference Mcgrath, Eyles, Pedersen, Anderson, Ko, Burne and Mortensen2010), childhood maltreatment (Varese et al., Reference Varese, Smeets, Drukker, Lieverse, Lataster, Viechtbauer and Bentall2012) and childhood trauma (Daruy-Filho, Brietzke, Lafer & Grassi-Oliveira, Reference Daruy-Filho, Brietzke, Lafer and Grassi-Oliveira2011; Morgan & Fisher, Reference Morgan and Fisher2007), to urbanicity (Vassos, Pedersen, Murray, Collier & Lewis, Reference Vassos, Pedersen, Murray, Collier and Lewis2012) and cannabis consumption (Hjorthøj, Posselt & Nordentoft, Reference Hjorthøj, Posselt and Nordentoft2021; Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). Familial high risk of severe mental disorders may be further explained by the environment in which children of parents with severe mental disorders are raised. Research suggests that a secure attachment between children and their caregivers is a protective factor against mental disorders (Rutter, Reference Rutter1985). However, little is known about the impact of environmental factors associated with being reared by a parent with a severe mental disorder.
It has proven difficult to differentiate between the environmental and genetic contribution to the familial high risk of severe mental disorders, one of the reasons being the presence of gene-environment interactions (Uher, Reference Uher2014). Individuals with a genetic risk are more vulnerable to environmental risk factors than those without a genetic risk (Uher, Reference Uher2014). Evidence suggests that individual differences, such as factors fostering resilience, influence the impact and sensitivity of environmental exposure (Collishaw et al., Reference Collishaw, Pickles, Messer, Rutter, Shearer and Maughan2007), which may be mediated through genetic factors to some degree (Uher, Reference Uher2009). However, not all individuals who either carry a genetic risk variant or are exposed to environmental risk factors or both will develop a severe mental disorder in adulthood (Van et al., Reference Van, Rutten, Myin-Germeys, Delespaul, Viechtbauer, Van and Mirjanic2014). Symptoms of vulnerability during childhood can be transitory (Gogtay et al., Reference Gogtay, Greenstein, Lenane, Clasen, Sharp, Gochman and Rapoport2007) but importantly we need to know more about which factors contributing to resilience.
Strengths and limitations
Our study has several major strengths, including the novelty of assessing sex-based differences in a large, same-aged, pre-pubertal sample with a familial risk of severe mental disorders. An advantage of assessing 7-year-old children before puberty is the limited influence of changing levels of hormones, which can contribute to sex differences (Kaczkurkin, Raznahan & Satterthwaite, Reference Kaczkurkin, Raznahan and Satterthwaite2019). Despite the difficulty in disentangling biological sex-based differences from those resulting from environmental and cultural influences, our study included both biological and environmental measures. A limitation of our study is that even though the assessors were blinded to group affiliation, they were not blinded to the sex of the child because the assessments were conducted face-to-face. Thus, scores could have been influenced by social expectations regarding the behavior of boys and girls. However, this influence would have been similar for the FHR groups and controls. Additionally, the cross-sectional nature of the study does not address the effect of development over time. We thus cannot dismiss the possibility that the effect of sex is transitorily and moreover, that girls might show impairments in domains which we have not assessed in this study. Since we did not assess the teacher-pupil relationship, we cannot rule out the possibility that the teachers knew about the familial high-risk disposition of the child, which potentially could have biased their ratings. Furthermore, the parents in the control group had no diagnoses of schizophrenia spectrum disorder or bipolar disorder but could potentially have other somatic and mental health disorders like in the general population. However, the advantage of this method, is the higher generalizability of the findings, in contrast to a more selected population.
To the best of our knowledge, this age-specific study is the first to assess sex-based differences among multiple domains in children with a familial risk of schizophrenia or bipolar disorder. We found that impairments within neurodevelopmental domains associated within FHR-SZ boys v. FHR-SZ girls were most evident among boys at age 7. Our results suggest heterogeneity in the development of FHR-children, with distinct sex characteristics among boys and girls with FHR-SZ and not children with FHR-BP. On a group level, boys with FHR-SZ had the highest proportion of neurodevelopmental impairments.
Acknowledgements
We thank all the children and their families who participated in the Danish High Risk and Resilience Study – VIA 7, to C. Tjott for the training and supervision in the assessment of Movement Assessment Battery for Children – Second Edition, to M. Skjærbæk, A. Ranning, H. Jensen, M. Melau, C. Gregersen, H. Stadsgaard, K. Kold Zahle and M. Toft for contributing to data collection; to C. Bøcker Pedersen and M. Giørtz Pedersen for retrieving the register extract; to J. Ohland, M. Chaine for help with data management and to P.B. Mortensen, T. Werge, D. Hougaard and A. Børglum for collaboration in iPSYCH. We thank Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
This work was supported by the Mental Health Services of the Capital Region of Denmark, the Independent Research Fund Denmark (#9039-00220B), the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH) (Grant nos. R102-A9118 and R155-2014-1724), Aarhus University, the Tryg Foundation and the Beatrice Surovell Haskell Fund for Child Mental Health Research of Copenhagen (Grant no. J.NR 11531). Sources of funding had no involvement in or influence on this work. The authors have no conflict of interest to declare.








